Bone marrow-derived macrophages from aged rats are more responsive to inflammatory stimuli
- PMID: 25890218
- PMCID: PMC4397943
- DOI: 10.1186/s12974-015-0287-7
Bone marrow-derived macrophages from aged rats are more responsive to inflammatory stimuli
Abstract
Background: Lipopolysaccharide (LPS) and interferon-γ (IFNγ) increase expression of tumour necrosis factor-α (TNFα) that characterizes the M1 activation state of macrophages. Whereas it is accepted that the immune system undergoes changes with age, there is inconsistency in the literature with respect to the impact of age on the response of macrophages to inflammatory stimuli. Here, we investigate the effect of age on the responsiveness of bone marrow-derived macrophages (BMDMs) to LPS and IFNγ. The context for addressing this question is that macrophages, which infiltrate the brain of aged animals, will encounter the neuroinflammatory environment that has been described with age.
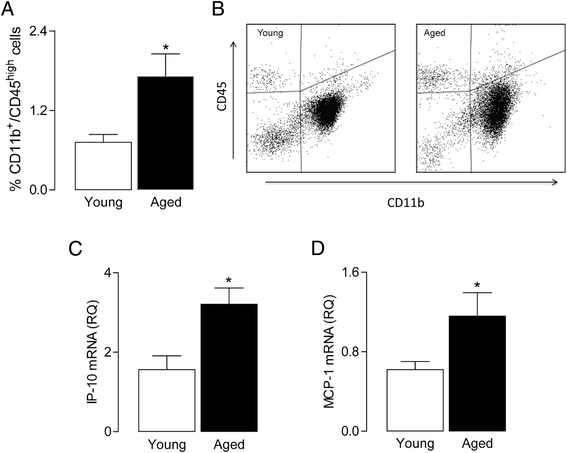
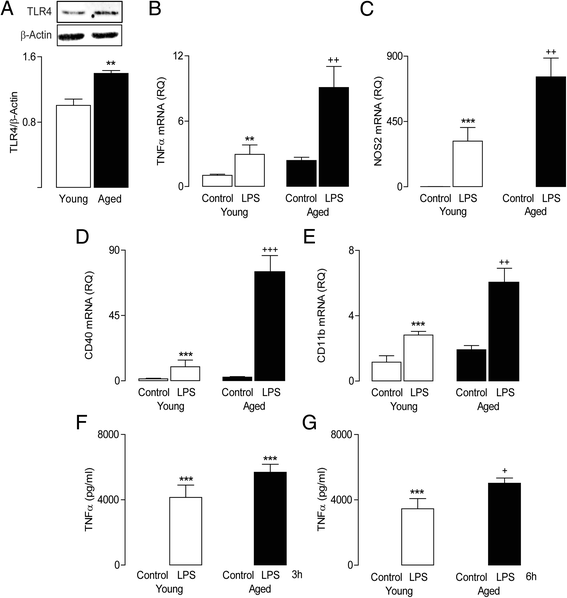
Methods: Brain tissue, prepared from young and aged rats, was assessed for expression of inflammatory markers by PCR and for evidence of infiltration of macrophages by flow cytometry. BMDMs were prepared from the long bones of young and aged rats, maintained in culture for 8 days and incubated in the presence or absence of LPS (100 ng/ml) or IFNγ (50 ng/ml). Cells were harvested and assessed for mRNA expression of markers of M1 activation including TNFα and NOS2, or for expression of IFNγR1 and TLR4 by western immunoblotting. To assess whether BMDMs induced glial activation, mixed glial cultures were incubated in the presence of conditioned media obtained from unstimulated BMDMs of young and aged rats and evaluated for expression of inflammatory markers.
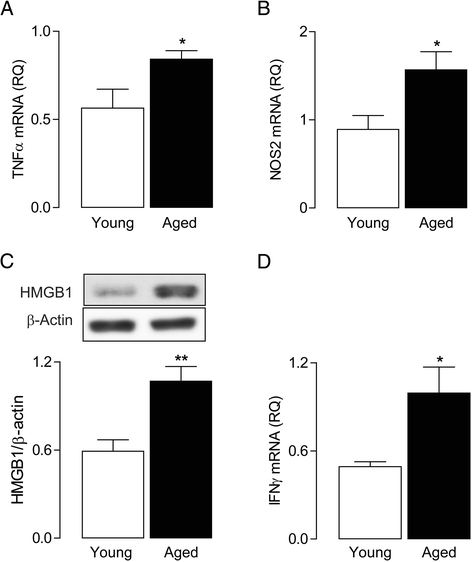
Results: Markers associated with M1 activation were expressed to a greater extent in BMDMs from aged rats in response to LPS and IFNγ, compared with cells from young rats. The increased responsiveness was associated with increases in IFNγ receptor (IFNγR) and Toll-like receptor 4 (TLR4). The data show that conditioned media from BMDMs of aged rats increased the expression of pro-inflammatory mediators in glial cells. Significantly, there was an age-related increase in macrophage infiltration into the brain, and this was combined with increased expression of IFNγ and the Toll-like receptor 4 agonist, high-mobility group protein B1 (HMGB1).
Conclusion: Exposure of infiltrating macrophages to the inflammatory microenvironment that develops in the brain with age is likely to contribute to a damaging cascade that negatively impacts neuronal function.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

