Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs
- PMID: 25890595
- PMCID: PMC4445729
- DOI: 10.22203/ecm.v029a17
Fact versus artifact: avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs
Abstract
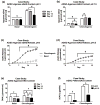
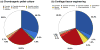
The 1,9-dimethylmethylene blue (DMMB) assay is widely used to quantify sulfated glycosaminoglycan (sGAG) contents of engineered tissues, culture media, tissue samples and bodily fluids, but the assay is subject to interference from polyanions such as hyaluronic acid (HA), DNA and RNA. We examined whether specific combinations of dye pH and absorbance wavelength could minimize non-sGAG artifacts without compromising DMMB assay sensitivity. HA and DNA solutions generated substantial signal at pH 3 but not at pH 1.5. Reducing dye pH did not significantly alter sGAG measurements for normal cartilage and meniscus tissues, but eliminated anomalously high apparent sGAG contents for enzymatically isolated chondrocytes, adipose-derived stem cell (ADSC)-agarose constructs and ADSC pellets. In a cartilage tissue-engineering case study, pH 3 dye indicated high apparent sGAG readings throughout culture in both basal and chondrogenic media, with a marked decline between day 14 and 21 for chondrogenic constructs. The pH 1.5 dye, however, indicated minimal sGAG accumulation in basal medium and stable sGAG content throughout culture in chondrogenic medium. As it is often difficult to know a priori whether all groups in a study will have sGAG contents high enough to overwhelm artifacts, we recommend modifying the standard DMMB assay to reduce the risk of spurious findings in tissue engineering and clinical research. Specifically, we recommend shifting to a pH 1.5 DMMB dye and basing quantification on the absorbance difference between 525 nm (µ peak) and 595 nm (β peak) to compensate for the moderate loss of sensitivity associated with reducing the dye pH.
Figures

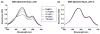


References
-
- Barbosa I, Garcia S, Barbier-Chassefiere V, Caruelle JP, Martelly I, Papy-Garcia D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology. 2003;13:647–653. - PubMed
-
- Billington CJ. Cartilage proteoglycan release assay. Methods Mol Biol. 2001;151:451–456. - PubMed
-
- Bitter T, Muir HM. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330–334. - PubMed
-
- Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161:103–108. - PubMed
-
- de Jong JG, Heijs WM, Wevers RA. Mucopolysaccharidoses screening: dimethylmethylene blue versus alcian blue. Ann Clin Biochem. 1994;31 (Pt 3):267–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials