Microtopographical features generated by photopolymerization recruit RhoA/ROCK through TRPV1 to direct cell and neurite growth
- PMID: 25890710
- PMCID: PMC4405664
- DOI: 10.1016/j.biomaterials.2015.02.057
Microtopographical features generated by photopolymerization recruit RhoA/ROCK through TRPV1 to direct cell and neurite growth
Abstract
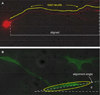
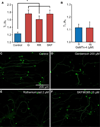
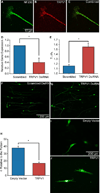
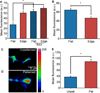
Cell processes, including growth cones, respond to biophysical cues in their microenvironment to establish functional tissue architecture and intercellular networks. The mechanisms by which cells sense and translate biophysical cues into directed growth are unknown. We used photopolymerization to fabricate methacrylate platforms with patterned microtopographical features that precisely guide neurite growth and Schwann cell alignment. Pharmacologic inhibition of the transient receptor potential cation channel subfamily V member 1 (TRPV1) or reduced expression of TRPV1 by RNAi significantly disrupts neurite guidance by these microtopographical features. Exogenous expression of TRPV1 induces alignment of NIH3T3 fibroblasts that fail to align in the absence of TRPV1, further implicating TRPV1 channels as critical mediators of cellular responses to biophysical cues. Microtopographic features increase RhoA activity in growth cones and in TRPV1-expressing NIH3T3 cells. Further, Rho-associated kinase (ROCK) phosphorylation is elevated in growth cones and neurites on micropatterned surfaces. Inhibition of RhoA/ROCK by pharmacological compounds or reduced expression of either ROCKI or ROCKII isoforms by RNAi abolishes neurite and cell alignment, confirming that RhoA/ROCK signaling mediates neurite and cell alignment to microtopographic features. These studies demonstrate that microtopographical cues recruit TRPV1 channels and downstream signaling pathways, including RhoA and ROCK, to direct neurite and cell growth.
Keywords: Cell signaling; Micropatterning; Nerve guide; Photopolymerization; Spiral ganglion neuron; Surface topography.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Song H, Poo M. The cell biology of neuronal navigation. Nat Cell Biol. 2001;3:E81–E88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

