Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty
- PMID: 25890715
- PMCID: PMC4405663
- DOI: 10.1016/j.biomaterials.2015.02.092
Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty
Abstract
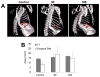
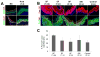
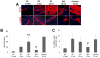
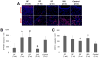
Surgical management of long-gap esophageal defects with autologous gastrointestinal tissues is frequently associated with adverse complications including organ dysmotility, dysphagia, and donor site morbidity. In order to develop alternative graft options, bi-layer silk fibroin (SF) scaffolds were investigated for their potential to support functional tissue regeneration in a rodent model of esophageal repair. Onlay esophagoplasty was performed with SF matrices (N = 40) in adult rats for up to 2 m of implantation. Parallel groups consisted of animals implanted with small intestinal submucosa (SIS) scaffolds (N = 22) or sham controls receiving esophagotomy alone (N = 20). Sham controls exhibited a 100% survival rate while rats implanted with SF and SIS scaffolds displayed respective survival rates of 93% and 91% prior to scheduled euthanasia. Animals in each experimental group were capable of solid food consumption following a 3 d post-op liquid diet and demonstrated similar degrees of weight gain throughout the study period. End-point μ-computed tomography at 2 m post-op revealed no evidence of contrast extravasation, fistulas, strictures, or diverticula in any of the implant groups. Ex vivo tissue bath studies demonstrated that reconstructed esophageal conduits supported by both SF and SIS scaffolds displayed contractile responses to carbachol, KCl and electrical field stimulation while isoproterenol produced tissue relaxation. Histological (Masson's trichrome and hematoxylin and eosin) and immunohistochemical (IHC) evaluations demonstrated both implant groups produced de novo formation of skeletal and smooth muscle bundles positive for contractile protein expression [fast myosin heavy chain (MY32) and α-smooth muscle actin (α-SMA)] within the graft site. However, SF matrices promoted a significant 4-fold increase in MY32+ skeletal muscle and a 2-fold gain in α-SMA+ smooth muscle in comparison to the SIS cohort as determined by histomorphometric analyses. A stratified squamous, keratinized epithelium expressing cytokeratin 5 and involucrin proteins was also present at 2 m post-op in all experimental groups. De novo innervation and vascularization were evident in all regenerated tissues indicated by the presence of synaptophysin (SYP38)+ boutons and vessels lined with CD31 expressing endothelial cells. In respect to SIS, the SF group supported a significant 4-fold increase in the density of SYP38+ boutons within the implant region. Evaluation of host tissue responses revealed that SIS matrices elicited chronic inflammatory reactions and severe fibrosis throughout the neotissues, in contrast to SF scaffolds. The results of this study demonstrate that bi-layer SF scaffolds represent promising biomaterials for onlay esophagoplasty, capable of producing superior regenerative outcomes in comparison to conventional SIS scaffolds.
Keywords: Epithelium; Muscle; Scaffold; Silk; Wound healing.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures





References
-
- Shen KR, Austen WG, Jr, Mathisen DJ. Use of a prefabricated pectoralis major muscle flap and pedicled jejunal interposition graft for salvage esophageal reconstruction after failed gastric pull-up and colon interposition. J Thorac Cardiovasc Surg. 2008;135:1186–7. - PubMed
-
- Burgos L, Barrena S, Andrés AM, Martínez L, Hernández F, Olivares P, et al. Colonic interposition for esophageal replacement in children remains a good choice: 33-year median follow-up of 65 patients. J Pediatr Surg. 2010;45:341–5. - PubMed
-
- Leonard GD, McCaffrey JA, Maher M. Optimal therapy for oesophageal cancer. Cancer Treat Rev. 2003;29:275–82. - PubMed
-
- Greene CL, DeMeester SR, Worrell SG, Oh DS, Hagen JA, DeMeester TR. Alimentary satisfaction, gastrointestinal symptoms, and quality of life 10 or more years after esophagectomy with gastric pull-up. J Thorac Cardiovasc Surg. 2014;147:909–14. - PubMed
-
- Romeo C, Bonanno N, Baldari S, Centorrino A, Scalfari G, Antonuccio P, et al. Gastric motility disorders in patients operated on for esophageal atresia and tracheoesophageal fistula: long term evaluation. J Pediatr Surg. 2000;35:740–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

