Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials
- PMID: 25890798
- PMCID: PMC4760351
- DOI: 10.1016/S2213-2600(15)00093-4
Association of hospital admission and forced vital capacity endpoints with survival in patients with idiopathic pulmonary fibrosis: analysis of a pooled cohort from three clinical trials
Abstract
Background: Mortality is an impractical primary endpoint for clinical trials in patients with idiopathic pulmonary fibrosis who have mild-to-moderate physiological impairment because event rates are low. Change in forced vital capacity (FVC) is widely accepted as a surrogate for mortality and is the most common primary endpoint in clinical trials for this disorder. Use of hospital admission as a predictor for mortality, independent of FVC decline, has not been well defined. We aimed to ascertain the independent and combined association of hospital admission and at least a 10% decrease in FVC with all-cause mortality.
Methods: We did a pooled cohort study of 517 patients with idiopathic pulmonary fibrosis from three IPFnet multicentre randomised controlled trials. We compared the incidence of non-elective hospital admission and a 10% or greater reduction in FVC across strata of baseline physiological impairment. We used Cox proportional-hazards models to assess the risk of all-cause mortality associated with these surrogate events, occurring up to a predefined landmark timepoint. The three studies are registered at ClinicalTrials.gov, numbers NCT00650091, NCT00517933, and NCT00957242.
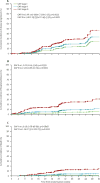
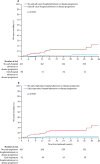
Findings: Seven patients died before the landmark timepoint. Of the 510 patients remaining, 38 (7%) were admitted to hospital up to the predefined timepoint and 58 (11%) had a categorical decrease in FVC of at least 10%. Most patients admitted to hospital did not have a 10% or greater decrease in FVC (30 vs eight). Both surrogate events were associated with subsequent time to death from any cause (hazard ratio [HR] for admission 4·05, 95% CI 1·36-12·11 vs HR for 10% or greater decline in FVC 4·68, 1·83-11·99). When causes of hospital admission were considered, only respiratory events were associated with mortality (5·97, 1·81-19·74).
Interpretation: Hospital admission might be an appropriate component of a clinically meaningful composite endpoint that improves the feasibility of clinical trials in idiopathic pulmonary fibrosis. Further studies are needed to refine the most appropriate definition of hospital admission for future trials.
Funding: US National Heart, Lung, and Blood Institute (NHLBI), and The Cowlin Family Fund at the Chicago Community Trust.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
HRC reports personal fees from Bayer, Biogen, Boehringer Ingelheim, FibroGen, Genentech, Gilead, Genoa, Inter Mune, Pfizer, Promedior, and Moerae Matrix, outside the submitted work. KKB reports grants from NIH/NHLBI during the conduct of the study, grants from NIH/NHLBI outside the submitted work, grants and personal fees from Actelion, Amgen and Gilead outside the submitted work, personal fees from Almiral, Altitude Pharma, Astra Zeneca, Bayer, Biogen/Stromedix, Boehringer Ingelheim, Celgene, Centocor, Fibrogen, Galecto, Glaxo Smith Kline, MedImmune, Novartis, Pfizer, Promedior, Roche/Genentech, Sanofi/Genzyme, and Veracyte outside the submitted work, and non-financial relationships with Bristol-Myers Squibb, GeNO, Genoa, Mesoblast, and Moerae outside the submitted work. KRF reports grants from NIH/NHLBI during the conduct of the study, board membership and personal fees from Boehringer Ingelheim outside the submitted work, personal fees from Fibrogen, Genentech, Gilead, Ikaria, Immune Works, Med Immune, Novartis, Takeda, Vertex, Veracyte, Roche, the Pulmonary Fibrosis Foundation, and UpToDate outside the submitted work, and institutional fees from Immune Works, Inter Mune and Bristol-Myers Squibb outside the submitted work. TEK reports personal fees from Inter Mune, Immune Works, Boehringer Ingelheim, Glaxo Smith Kline, Daiichi Sankyo, and UpToDate during the conduct of the study but outside the submitted work. SMP reports grants from Bristol-Myers Squibb and Gilead, outside the submitted work. GR reports grants from NIH/NHLBI during the conduct of the study, consultancies for Biogen, Boehringer Ingelheim, Centocor, Fibrogen, Gilead, Inter Mune, Actelion, Promedior, and Veracyte outside the submitted work, and data safety monitoring board membership for MedImmune outside the submitted work. KJA reports grants from NIH/NHLBI during the conduct of the study. FJM reports grants from NIH/NHLBI during the conduct of the study, steering committee membership for studies funded by Bayer, Centocor, Gilead and Promediorduring the conduct of the study but outside the submitted work, personal fees from Ikaria, Genentech, Nycomed/Takeda, Pfizer, Vertex, MedScape, Stromedix/Biogen, Axon Communications, Johnson & Johnson, Genzyme, the National Association for Continuing Education, Boehringer Ingelheim, Veracyte, the American Thoracic Society, Inova Health System, Spectrum Health System, and the University of Texas-Southwestern during the conduct of the study but outside the submitted work, and personal fees from Forest, Janssen, Glaxo Smith Kline, NycoMed/Takeda, Genentech, Actelion, Amgen, Astra Zeneca, Carden Jennings, CSA Medical, Ikaria/Bellerophon, Merck, Pearl, Roche, Sudler& Hennessey, CME Incite, the Center for Healthcare Education, Miller Medical, Paradigm, Peer Voice, Projects in Knowledge, Bayer, Forest, Grey Healthcare, Merion, Informa, Annenberg, UpToDate, MedScape, the American College of Chest Physicians, Inova Health System, St. John’s Hospital, St. Mary’s Hospital, the University of Illinois-Chicago, the University of Virginia, and Wayne State University, outside the submitted work. All other authors declare that they have no competing interests.
Figures



Comment in
-
Efficacy endpoints for idiopathic pulmonary fibrosis trials.Lancet Respir Med. 2015 May;3(5):335-7. doi: 10.1016/S2213-2600(15)00146-0. Epub 2015 Apr 15. Lancet Respir Med. 2015. PMID: 25890799 No abstract available.
References
-
- King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. The New England journal of medicine. 2014;370(22):2083–92. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. The New England journal of medicine. 2014;370(22):2071–82. - PubMed
-
- du Bois RM. Strategies for treating idiopathic pulmonary fibrosis. Nature reviews Drug discovery. 2010;9(2):129–40. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
- U10HL80370/HL/NHLBI NIH HHS/United States
- U10HL80571/HL/NHLBI NIH HHS/United States
- K24 HL111316/HL/NHLBI NIH HHS/United States
- 5T32HL007538/HL/NHLBI NIH HHS/United States
- U10HL80510/HL/NHLBI NIH HHS/United States
- T32 HL007538/HL/NHLBI NIH HHS/United States
- U10 HL080413/HL/NHLBI NIH HHS/United States
- U10 HL080513/HL/NHLBI NIH HHS/United States
- U10 HL080543/HL/NHLBI NIH HHS/United States
- U10HL80685/HL/NHLBI NIH HHS/United States
- U10HL80543/HL/NHLBI NIH HHS/United States
- U10 HL080274/HL/NHLBI NIH HHS/United States
- U10HL80513/HL/NHLBI NIH HHS/United States
- U10 HL080371/HL/NHLBI NIH HHS/United States
- U10HL80413/HL/NHLBI NIH HHS/United States
- U10HL80274/HL/NHLBI NIH HHS/United States
- U10HL80383/HL/NHLBI NIH HHS/United States
- U10 HL080383/HL/NHLBI NIH HHS/United States
- U10 HL080370/HL/NHLBI NIH HHS/United States
- U10 HL080411/HL/NHLBI NIH HHS/United States
- U10HL080509/HL/NHLBI NIH HHS/United States
- U10HL80371/HL/NHLBI NIH HHS/United States
- U10HL80509/HL/NHLBI NIH HHS/United States
- U10 HL080509/HL/NHLBI NIH HHS/United States
- U10 HL080571/HL/NHLBI NIH HHS/United States
- U10HL80411/HL/NHLBI NIH HHS/United States
- U10 HL080685/HL/NHLBI NIH HHS/United States
- U10 HL080510/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

