The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis
- PMID: 25891079
- PMCID: PMC4464991
- DOI: 10.1039/c4mt00332b
The role of transition metal transporters for iron, zinc, manganese, and copper in the pathogenesis of Yersinia pestis
Abstract
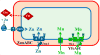
Yersinia pestis, the causative agent of bubonic, septicemic and pneumonic plague, encodes a multitude of Fe transport systems. Some of these are defective due to frameshift or IS element insertions, while others are functional in vitro but have no established role in causing infections. Indeed only 3 Fe transporters (Ybt, Yfe and Feo) have been shown to be important in at least one form of plague. The yersiniabactin (Ybt) system is essential in the early dermal/lymphatic stages of bubonic plague, irrelevant in the septicemic stage, and critical in pneumonic plague. Two Mn transporters have been characterized (Yfe and MntH). These two systems play a role in bubonic plague but the double yfe mntH mutant is fully virulent in a mouse model of pneumonic plague. The same in vivo phenotype occurs with a mutant lacking two (Yfe and Feo) of four ferrous transporters. A role for the Ybt siderophore in Zn acquisition has been revealed. Ybt-dependent Zn acquisition uses a transport system completely independent of the Fe-Ybt uptake system. Together Ybt components and ZnuABC play a critical role in Zn acquisition in vivo. Single mutants in either system retain high virulence in a mouse model of septicemic plague while the double mutant is completely avirulent.
Figures

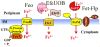

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

