The Redox Code
- PMID: 25891126
- PMCID: PMC4580308
- DOI: 10.1089/ars.2015.6247
The Redox Code
Abstract
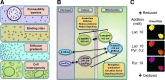
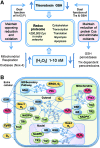
Significance: The redox code is a set of principles that defines the positioning of the nicotinamide adenine dinucleotide (NAD, NADP) and thiol/disulfide and other redox systems as well as the thiol redox proteome in space and time in biological systems. The code is richly elaborated in an oxygen-dependent life, where activation/deactivation cycles involving O₂ and H₂O₂ contribute to spatiotemporal organization for differentiation, development, and adaptation to the environment. Disruption of this organizational structure during oxidative stress represents a fundamental mechanism in system failure and disease.
Recent advances: Methodology in assessing components of the redox code under physiological conditions has progressed, permitting insight into spatiotemporal organization and allowing for identification of redox partners in redox proteomics and redox metabolomics.
Critical issues: Complexity of redox networks and redox regulation is being revealed step by step, yet much still needs to be learned.
Future directions: Detailed knowledge of the molecular patterns generated from the principles of the redox code under defined physiological or pathological conditions in cells and organs will contribute to understanding the redox component in health and disease. Ultimately, there will be a scientific basis to a modern redox medicine.
Figures



References
-
- Abate C, Patel L, Rauscher FJ, 3rd, and Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science 249: 1157–1161, 1990 - PubMed
-
- Bass J. Circadian topology of metabolism. Nature 491: 348–356, 2012 - PubMed
-
- Beck MA, Nelson HK, Shi Q, Van Dael P, Shiffrin EJ, Blum S, Barclay D, and Levander OA. Selenium deficiency increases the pathology of an influenza infection. FASEB J 15: 1481–1483, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

