Romidepsin in peripheral and cutaneous T-cell lymphoma: mechanistic implications from clinical and correlative data
- PMID: 25891346
- PMCID: PMC4675455
- DOI: 10.1111/bjh.13400
Romidepsin in peripheral and cutaneous T-cell lymphoma: mechanistic implications from clinical and correlative data
Abstract
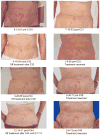
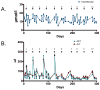
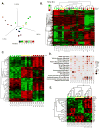
Romidepsin is an epigenetic agent approved for the treatment of patients with cutaneous or peripheral T-cell lymphoma (CTCL and PTCL). Here we report data in all patients treated on the National Cancer Institute 1312 trial, demonstrating long-term disease control and the ability to retreat patients relapsing off-therapy. In all, 84 patients with CTCL and 47 with PTCL were enrolled. Responses occurred early, were clinically meaningful and of very long duration in some cases. Notably, patients with PTCL receiving romidepsin as third-line therapy or later had a comparable response rate (32%) of similar duration as the total population (38%). Eight patients had treatment breaks of 3.5 months to 10 years; in four of six patients, re-initiation of treatment led to clear benefit. Safety data show slightly greater haematological and constitutional toxicity in PTCL. cDNA microarray studies show unique individual gene expression profiles, minimal overlap between patients, and both induction and repression of gene expression that reversed within 24 h. These data argue against cell death occurring as a result of an epigenetics-mediated gene induction programme. Together this work supports the safety and activity of romidepsin in T-cell lymphoma, but suggests a complex mechanism of action.
Trial registration: ClinicalTrials.gov NCT00007345.
Keywords: T-cell lymphoma; chromatin; epigenetic therapy; histone deacetylase inhibitor; romidepsin.
© 2015 John Wiley & Sons Ltd.
Conflict of interest statement
Drs. Bates, Piekarz, Prince, Kirschbaum, Allen, Zain, Geskin, Joske, Popplewell and Cowen received funding from a CRADA between the NIH and Celgene Corporation. Drs. Nichols, Zain and Kennedy were employed by Glouchester Pharmaceuticals/Celgene Corporation. The other authors declare no conflict of interest.
Figures


References
-
- Bates S, Zhan Z, Steadman K, Obrzut T, Luchenko V, Frye R, Robey R, Turner M, Gardner E, Figg W, Steinberg S, Ling A, Fojo T, To K, Piekarz R. Laboratory correlates for a phase II trial of romidepsin in cutaneous and peripheral T-cell lymphoma. British Journal of Haematology. 2010;148:256–267. - PMC - PubMed
-
- Bhalla S, Balasubramanian S, David K, Sirisawad M, Buggy J, Mauro L, Prachand S, Miller R, Gordon LI, Evens AM. PCI-24781 induces caspase and reactive oxygen species-dependent apoptosis through NF-kappaB mechanisms and is synergistic with bortezomib in lymphoma cells. Clinical Cancer Research. 2009;15:3354–3365. - PMC - PubMed
-
- Cabell C, Bates S, Piekarz R, Whittaker S, Kim YH, Currie M, Godfrey CJ, Schoonmaker C, Nichols J, Nix D, Burris HA. Systematic Assessment of Potential Cardiac Effects of the Novel Histone Deacetylase (HDAC) Inhibitor Romidepsin. Blood (American Society of Hematology Annual Meeting Abstracts) 2009;114:3709.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

