Paclitaxel impairs adipose stem cell proliferation and differentiation
- PMID: 25891676
- PMCID: PMC4442730
- DOI: 10.1016/j.jss.2015.03.026
Paclitaxel impairs adipose stem cell proliferation and differentiation
Abstract
Background: Cancer patients with chemotherapy-induced immunosuppression have poor surgical site wound healing. Prior literature supports the use of human adipose-derived stem cell (hASC) lipoinjection to improve wound healing. It has been established that multipotent hASCs facilitate neovascularization, accelerate epithelialization, and quicken wound closure in animal models. Although hASC wound therapy may benefit surgical cancer patients, the chemotherapeutic effects on hASCs are unknown. We hypothesized that paclitaxel, a chemotherapeutic agent, impairs hASC growth, multipotency, and induces apoptosis.
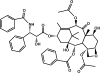
Methods: hASCs were isolated and harvested from consented, chemotherapy and radiation naive patients. Growth curves, MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide), and EdU (5-ethynyl-2-deoxyguridine) assays measured cytotoxicity and proliferation. Oil Red O stain, Alizarin Red stain, matrigel tube formation assay, and quantitative polymerase chain reaction analyzed hASC differentiation. Annexin V assay measured apoptosis. Immunostaining and Western blot determined tumor necrosis factor α (TNF-α) expression.
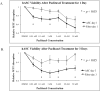
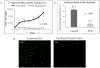
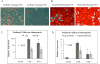
Results: hASCs were selectively more sensitive to paclitaxel (0.01-30 μM) than fibroblasts (P < 0.05). After 12 d, paclitaxel caused hASC growth arrest, whereas control hASCs proliferated (P = 0.006). Paclitaxel caused an 80.6% reduction in new DNA synthesis (P < 0.001). Paclitaxel severely inhibited endothelial differentiation and capillary-like tube formation. Differentiation markers, lipoprotein lipase (adipogenic), alkaline phosphatase (osteogenic), CD31, and van Willebrand factor (endothelial), were significantly decreased (all P < 0.05) confirming paclitaxel impaired differentiation. Paclitaxel was also found to induce apoptosis and TNF-α was upregulated in paclitaxel-treated hASCs (P < 0.001).
Conclusions: Paclitaxel is more cytotoxic to hASCs than fibroblasts. Paclitaxel inhibits hASC proliferation, differentiation, and induces apoptosis, possibly through the TNF-α pathway. Paclitaxel's severe inhibition of endothelial differentiation indicates neovascularization disruption, possibly causing poor wound healing in cancer patients receiving chemotherapy.
Keywords: Cancer therapy; Chronic wounds; Human adipose–derived stem cells; Neovascularization; Paclitaxel; Wound healing.
Copyright © 2015 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures




References
-
- Springfield DS. Surgical wound healing. Cancer Treat Res. 1993;67:81–98. - PubMed
-
- Drank DB, Oishi SN. Wound healing considerations in chemotherapy and radiation therapy. Clin Plast Surg. 1995;22(1):31–7. - PubMed
-
- Cornell K, Waters DJ. Impaired wound healing in the cancer patient: effects of cytotoxic therapy and pharmacologic modulation by growth factors. Vet Clin North Am Small Anim Pract. 1995;25(1):111–31. - PubMed
-
- Ashraf A, Lee PH, Kim K, Zaporojan V, Bonassar L, Valentini R, et al. Effect of sustained-release PDGF and TGF-beta on cyclophosphamide induced impaired wound healing. Plast Reconstr Surg. 2009;124:1118–24. - PubMed
-
- Obara K, Ishihara M, Fujita M, Kanatani Y, Hattori H, Matsui T, et al. Acceleration of wound healing in healing-imparied db/db mice wit ha photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2. Wound Repair Regen. 2005;13:390–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

