Immunosurveillance of the liver by intravascular effector CD8(+) T cells
- PMID: 25892224
- PMCID: PMC11630812
- DOI: 10.1016/j.cell.2015.03.005
Immunosurveillance of the liver by intravascular effector CD8(+) T cells
Abstract
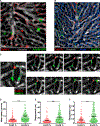
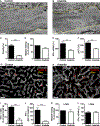
Effector CD8(+) T cells (CD8 TE) play a key role during hepatotropic viral infections. Here, we used advanced imaging in mouse models of hepatitis B virus (HBV) pathogenesis to understand the mechanisms whereby these cells home to the liver, recognize antigens, and deploy effector functions. We show that circulating CD8 TE arrest within liver sinusoids by docking onto platelets previously adhered to sinusoidal hyaluronan via CD44. After the initial arrest, CD8 TE actively crawl along liver sinusoids and probe sub-sinusoidal hepatocytes for the presence of antigens by extending cytoplasmic protrusions through endothelial fenestrae. Hepatocellular antigen recognition triggers effector functions in a diapedesis-independent manner and is inhibited by the processes of sinusoidal defenestration and capillarization that characterize liver fibrosis. These findings reveal the dynamic behavior whereby CD8 TE control hepatotropic pathogens and suggest how liver fibrosis might reduce CD8 TE immune surveillance toward infected or transformed hepatocytes.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures





References
-
- Ando K, Guidotti LG, Cerny A, Ishikawa T, and Chisari FV (1994). CTL access to tissue antigen is restricted in vivo. J. Immunol. 153, 482–488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

