Sustained proliferation in cancer: Mechanisms and novel therapeutic targets
- PMID: 25892662
- PMCID: PMC4898971
- DOI: 10.1016/j.semcancer.2015.02.006
Sustained proliferation in cancer: Mechanisms and novel therapeutic targets
Abstract
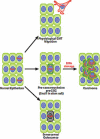
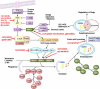
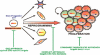
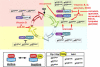
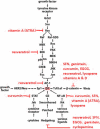
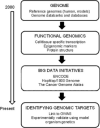
Proliferation is an important part of cancer development and progression. This is manifest by altered expression and/or activity of cell cycle related proteins. Constitutive activation of many signal transduction pathways also stimulates cell growth. Early steps in tumor development are associated with a fibrogenic response and the development of a hypoxic environment which favors the survival and proliferation of cancer stem cells. Part of the survival strategy of cancer stem cells may manifested by alterations in cell metabolism. Once tumors appear, growth and metastasis may be supported by overproduction of appropriate hormones (in hormonally dependent cancers), by promoting angiogenesis, by undergoing epithelial to mesenchymal transition, by triggering autophagy, and by taking cues from surrounding stromal cells. A number of natural compounds (e.g., curcumin, resveratrol, indole-3-carbinol, brassinin, sulforaphane, epigallocatechin-3-gallate, genistein, ellagitannins, lycopene and quercetin) have been found to inhibit one or more pathways that contribute to proliferation (e.g., hypoxia inducible factor 1, nuclear factor kappa B, phosphoinositide 3 kinase/Akt, insulin-like growth factor receptor 1, Wnt, cell cycle associated proteins, as well as androgen and estrogen receptor signaling). These data, in combination with bioinformatics analyses, will be very important for identifying signaling pathways and molecular targets that may provide early diagnostic markers and/or critical targets for the development of new drugs or drug combinations that block tumor formation and progression.
Keywords: Cancer hallmarks; Cancer stem cells; Natural products; Proliferation; Therapeutic targets.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- CA155686/CA/NCI NIH HHS/United States
- R01 AI076535/AI/NIAID NIH HHS/United States
- R01 CA131294/CA/NCI NIH HHS/United States
- U54 CA118638/CA/NCI NIH HHS/United States
- R21 CA155686/CA/NCI NIH HHS/United States
- C301/A14762/Cancer Research UK/United Kingdom
- R03DK089130/DK/NIDDK NIH HHS/United States
- R01 CA066971/CA/NCI NIH HHS/United States
- R03 DK089130/DK/NIDDK NIH HHS/United States
- K01DK077137/DK/NIDDK NIH HHS/United States
- K01 DK077137/DK/NIDDK NIH HHS/United States
- R01 CA109335-04A1/CA/NCI NIH HHS/United States
- R01CA131294/CA/NCI NIH HHS/United States
- R01 CA109335/CA/NCI NIH HHS/United States
- AI076535/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

