Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital
- PMID: 25892889
- PMCID: PMC4394100
- DOI: 10.3748/wjg.v21.i14.4373
Ipilimumab associated colitis: an IpiColitis case series at MedStar Georgetown University Hospital
Abstract
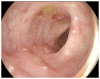
Although ipilimumab has been shown to improve survival in patients with metastatic melanoma and cause regression of metastatic renal cell carcinoma, the associated immune-related toxicities are of concern. The resultant T cell activation by this monoclonal antibody causes an increased immune response, which has been associated with many immune-regulated adverse effects. One of the most concerning effects is the development of colitis. Upwards to 8% of patients have been reported to develop colitis, with 5% being severe (Grades 3-4). While initial treatment of such adverse effects is generally comprised of supportive and symptomatic treatment, more severe cases warrant the use of high dose steroids. Furthermore, use of anti-TNF agents is usually reserved for those cases that prove to be refractory to steroids. We describe a systematic case review of seven patients who developed gastrointestinal symptoms following initiation of ipilimumab immunotherapy, and present the steps in their evaluation, treatment and outcomes at our institution.
Keywords: Colitis; Immune-regulated adverse effects; Immunology; Infliximab; Ipilimumab.
Figures



References
-
- Hanaizi Z, van Zwieten-Boot B, Calvo G, Lopez AS, van Dartel M, Camarero J, Abadie E, Pignatti F. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur J Cancer. 2012;48:237–242. - PubMed
-
- Graziani G, Tentori L, Navarra P. Ipilimumab: a novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res. 2012;65:9–22. - PubMed
-
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE). 2009-05-28, cited 2015-01-12; 80 screens. Available from: http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

