Structural requirements for bisphosphonate binding on hydroxyapatite: NMR study of bisphosphonate partial esters
- PMID: 25893039
- PMCID: PMC4394349
- DOI: 10.1021/ml5004603
Structural requirements for bisphosphonate binding on hydroxyapatite: NMR study of bisphosphonate partial esters
Abstract
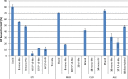
Eighteen different bisphosphonates, including four clinically used bisphosphonate acids and their phosphoesters, were studied to evaluate how the bisphosphonate structure affects binding to bone. Bisphosphonates with weak bone affinity, such as clodronate, could not bind to hydroxyapatite after the addition of one ester group. Medronate retained its ability to bind after the addition of one ester group, and hydroxy-bisphosphonates could bind even after the addition of two ester groups. Thus, several bisphosphonate esters are clearly bone binding compounds. The following conclusions about bisphosphonate binding emerge: (1) a hydroxyl group in the geminal carbon takes part in the binding process and increases the bisphosphonate's ability to bind to bone; (2) the bisphosphonate's ability to bind decreases when the amount of ester groups increases; and (3) the location of the ester groups affects the bisphosphonate's binding ability.
Keywords: Bisphosphonates; alendronate; etidronate; hydroxyapatite; nuclear magnetic resonance.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
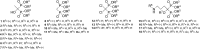

Similar articles
-
The Effects of Various Food Products on Bisphosphonate's Availability.Pharmaceutics. 2022 Mar 27;14(4):717. doi: 10.3390/pharmaceutics14040717. Pharmaceutics. 2022. PMID: 35456551 Free PMC article.
-
Comparative appraisal of clodronate, aspirin and dexamethasone as agents reducing alendronate-induced inflammation in a murine model.Basic Clin Pharmacol Toxicol. 2005 Oct;97(4):222-9. doi: 10.1111/j.1742-7843.2005.pto_138.x. Basic Clin Pharmacol Toxicol. 2005. PMID: 16176557
-
Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro.Materials (Basel). 2020 May 1;13(9):2086. doi: 10.3390/ma13092086. Materials (Basel). 2020. PMID: 32369961 Free PMC article.
-
Bisphosphonates: from the laboratory to the clinic and back again.Bone. 1999 Jul;25(1):97-106. doi: 10.1016/s8756-3282(99)00116-7. Bone. 1999. PMID: 10423031 Review.
-
The relationship between the chemistry and biological activity of the bisphosphonates.Bone. 2011 Jul;49(1):20-33. doi: 10.1016/j.bone.2011.03.774. Epub 2011 Apr 9. Bone. 2011. PMID: 21497677 Review.
Cited by
-
Hydroxy- and Amino-Phosphonates and -Bisphosphonates: Synthetic Methods and Their Biological Applications.Front Chem. 2022 Jun 1;10:890696. doi: 10.3389/fchem.2022.890696. eCollection 2022. Front Chem. 2022. PMID: 35721002 Free PMC article. Review.
-
Zoledronate alters natural progression of tissue-engineered vascular grafts.FASEB J. 2021 Oct;35(10):e21849. doi: 10.1096/fj.202001606RR. FASEB J. 2021. PMID: 34473380 Free PMC article.
-
Immobilized Bisphosphonates as Potential Inhibitors of Bioprosthetic Calcification: Effects on Various Xenogeneic Cardiovascular Tissues.Biomedicines. 2021 Dec 29;10(1):65. doi: 10.3390/biomedicines10010065. Biomedicines. 2021. PMID: 35052745 Free PMC article.
-
Research advances of nanomaterials for the acceleration of fracture healing.Bioact Mater. 2023 Aug 27;31:368-394. doi: 10.1016/j.bioactmat.2023.08.016. eCollection 2024 Jan. Bioact Mater. 2023. PMID: 37663621 Free PMC article. Review.
-
Synthesis of medronic acid monoesters and their purification by high-performance countercurrent chromatography or by hydroxyapatite.Beilstein J Org Chem. 2016 Oct 7;12:2145-2149. doi: 10.3762/bjoc.12.204. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 27829921 Free PMC article.
References
-
- Cremers S.; Papapoulos S. Pharmacology of bisphosphonates. Bone 2011, 49, 42–49. - PubMed
-
- Gralow J.; Tripathy D. Managing metastatic bone pain: the role of bisphosphonates. J. Pain Symptom Manage. 2007, 33, 462–472. - PubMed
-
- Clézardin P. Bisphosphonates’ antitumor activity: an unravelled side of a multifaceted drug class. Bone 2011, 48, 71–79. - PubMed
-
- Lin J. Bisphosphonates: a review of their pharmacokinetic properties. Bone 1996, 18, 75–85. - PubMed
-
- Nancollas G.; Tang R.; Phipps R.; Henneman Z.; Gulde S.; Wu W.; Mangood A.; Russell R.; Ebetino F. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 2006, 38, 617–627. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

