Synthetically defined glycoprotein vaccines: current status and future directions
- PMID: 25893089
- PMCID: PMC4396375
- DOI: 10.1039/c3sc50862e
Synthetically defined glycoprotein vaccines: current status and future directions
Abstract
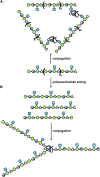
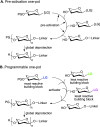
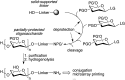
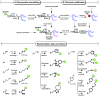
Primary examples in vaccine design have shown good levels of carbohydrate-specific antibody generation when raised using extracted or fully synthetic capsular polysaccharide glycans covalently coupled to a protein carrier. Herein, we cover recent clinical developments of carbohydrate-based vaccines and describe how novel cutting-edge methodology for the total synthesis of oligosaccharides and for the precise placement of carbohydrates at pre-determined sites within a protein may be used to further improve the safety and efficacy of glycovaccines.
Figures


References
-
- Avci F. Y., Kasper D. L. Annu. Rev. Immunol. 2010;28:107–130. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

