Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients
- PMID: 25893216
- PMCID: PMC4399775
- DOI: 10.1523/ENEURO.0006-14.2014
Altered Brain Structure and Function Correlate with Disease Severity and Pain Catastrophizing in Migraine Patients
Abstract
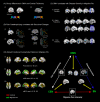
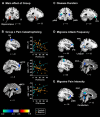
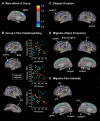
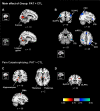
To investigate the neuroanatomical and functional brain changes in migraine patients relative to healthy controls, we used a combined analytical approach including voxel- and surface-based morphometry along with resting-state functional connectivity to determine whether areas showing structural alterations in patients also showed abnormal functional connectivity. Additionally, we wanted to assess whether these structural and functional changes were associated with group differences in pain catastrophizing and migraine-related disease variables in patients. We acquired T1-weighted anatomical and functional magnetic resonance imaging scans during rest in human subjects with a diagnosis of migraine and healthy controls. Structural analyses revealed greater left hippocampal gray matter volume and reduced cortical thickness in the left anterior midcingulate in patients compared with controls. We also observed negative associations between pain catastrophizing and migraine disease variables and gray matter in areas implicated in processing the sensory, affective, and cognitive aspects of pain in patients. Functional connectivity analyses showed that migraine patients displayed disrupted connectivity between default mode, salience, cognitive, visuospatial, and sensorimotor networks, which was associated with group differences in pain catastrophizing and migraine-related disease variables in patients. Together, our findings show widespread morphological and functional brain abnormalities in migraineurs in affective, cognitive, visual, and pain-related brain areas, which are associated with increased pain catastrophizing, disease chronicity, and severity of symptoms, suggesting that these structural and functional changes may be a consequence of repeated, long-term nociceptive signaling leading to increased pain sensitivity, mood disturbances, and maladaptive coping strategies to deal with unrelenting pain.
Keywords: chronic pain; gray matter; headache; intrinsic connectivity; neuroimaging; resting-state networks.
Conflict of interest statement
We thank the University of Maryland Magnetic Resonance Research Center, Dr. Rao Gullapalli and George Makris for their assistance with data collection. We would also like to express our gratitude to all the participants who took part in this study. The authors declare no competing financial interests.
Figures

References
-
- Archer DP, Roth SH (1997) Pharmacodynamics of thiopentone: nocifensive reflex threshold changes correlate with hippocampal electroencephalography. Br J Anaesth 79:744-749. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
