Trends in cardiac biomarker testing in China for patients with acute myocardial infarction, 2001 to 2011: China PEACE-retrospective AMI study
- PMID: 25893247
- PMCID: PMC4404305
- DOI: 10.1371/journal.pone.0122237
Trends in cardiac biomarker testing in China for patients with acute myocardial infarction, 2001 to 2011: China PEACE-retrospective AMI study
Abstract
Objectives: To describe trends in the availability of biomarker testing in Chinese hospitals and how practice complies with established standards for the diagnosis of acute myocardial infarction (AMI).
Background: Cardiac biomarker testing is standard in high-income countries, but little is known about the availability and use of cardiac biomarker testing in low- and middle-income countries (LMICs) such as China.
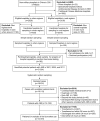
Methods: Based on a nationally representative sample of Chinese hospitals in 2001, 2006 and 2011, we describe the temporal trends and regional differences in the hospital capability and rates of use of cardiac biomarker testing, as well as the variation in use across hospitals with testing capability, for patients labeled with the diagnosis of AMI.
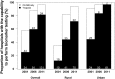
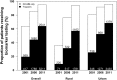
Results: We sampled 175 hospitals (162 participated in the study) and 18,631 AMI admissions. 14,370 patients were included in analysis of biomarker use. The proportion of hospitals with biomarker testing capability was 57.4% in 2001 (25.0% troponin and 32.4% creatine kinase MB fraction (CK-MB) only) and 96.3% (81.4% troponin and 14.9% CK-MB only) in 2011. The proportion of hospitals with troponin testing capability in 2011 was significantly higher in urban compared with rural hospitals (96.8% vs. 71.4%, p< 0.001). In 2011, only 55.9% of hospitals with troponin testing capability (71 out of 127 hospitals) used the assay for more than 80% of their patients with AMI. Among hospitals with either biomarker testing capability, there was marked variation in use in both rural (from 7.1% to 100.0% of patients) and urban hospitals (from 57.9% to 100.0% of patients). In 2011, 36.1% of the patients with AMI did not have troponin tested and 4.9% did not have either biomarker measured.
Conclusions: The recommended biomarker tests for AMI diagnosis are not universally available and the testing is not consistently applied when it is available in China.
Trial registration: ClinicalTrials.gov NCT01624883.
Conflict of interest statement
Figures

References
-
- Mathers CD, Fat DM, Boerma J. The global burden of disease: 2004 update: World Health Organization; 2008.
-
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013; 127:e362–425. 10.1161/CIR.0b013e3182742cf6 - DOI - PubMed
-
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007; 116: e148–304. - PubMed
-
- Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J. 2000; 21: 1502–1513. - PubMed
-
- Jaffe AS, Ravkilde J, Roberts R, Naslund U, Apple FS, Galvani M, et al. It's time for a change to a troponin standard. Circulation. 2000; 102: 1216–1220. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

