The epigenetic regulators CBP and p300 facilitate leukemogenesis and represent therapeutic targets in acute myeloid leukemia
- PMID: 25893291
- PMCID: PMC4729186
- DOI: 10.1038/onc.2015.92
The epigenetic regulators CBP and p300 facilitate leukemogenesis and represent therapeutic targets in acute myeloid leukemia
Abstract
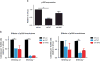
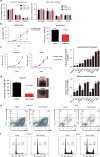
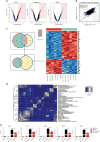
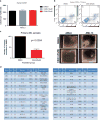
Growing evidence links abnormal epigenetic control to the development of hematological malignancies. Accordingly, inhibition of epigenetic regulators is emerging as a promising therapeutic strategy. The acetylation status of lysine residues in histone tails is one of a number of epigenetic post-translational modifications that alter DNA-templated processes, such as transcription, to facilitate malignant transformation. Although histone deacetylases are already being clinically targeted, the role of histone lysine acetyltransferases (KAT) in malignancy is less well characterized. We chose to study this question in the context of acute myeloid leukemia (AML), where, using in vitro and in vivo genetic ablation and knockdown experiments in murine models, we demonstrate a role for the epigenetic regulators CBP and p300 in the induction and maintenance of AML. Furthermore, using selective small molecule inhibitors of their lysine acetyltransferase activity, we validate CBP/p300 as therapeutic targets in vitro across a wide range of human AML subtypes. We proceed to show that growth retardation occurs through the induction of transcriptional changes that induce apoptosis and cell-cycle arrest in leukemia cells and finally demonstrate the efficacy of the KAT inhibitors in decreasing clonogenic growth of primary AML patient samples. Taken together, these data suggest that CBP/p300 are promising therapeutic targets across multiple subtypes in AML.
Conflict of interest statement
PA Cole is a cofounder, equity holder and paid consultant for Acylin Therapeutics which is developing p300 HAT inhibitors.
Figures


References
-
- Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. - PubMed
-
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367:647–657. - PubMed
-
- Blobel GA. CREB-binding protein and p300: molecular integrators of hematopoietic transcription. Blood. 2000;95:745–755. - PubMed
-
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

