MicroRNAs in apoptosis, autophagy and necroptosis
- PMID: 25893379
- PMCID: PMC4496162
- DOI: 10.18632/oncotarget.3523
MicroRNAs in apoptosis, autophagy and necroptosis
Abstract
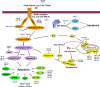
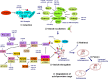
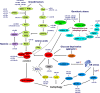
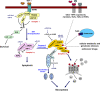
MicroRNAs (miRNAs) are endogenous 22 nt non-coding RNAs that target mRNAs for cleavage or translational repression. Numerous miRNAs regulate programmed cell death including apoptosis, autophagy and necroptosis. We summarize how miRNAs regulate apoptotic, autophagic and necroptotic pathways and cancer progression. We also discuss how miRNAs link different types of cell death.
Keywords: apoptosis; autophagy; cancer progression; microRNA; necroptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Png KJ, Halberg N, Yoshida M, Tavazoie SF. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

