Integrated compensatory network is activated in the absence of NCC phosphorylation
- PMID: 25893600
- PMCID: PMC4463200
- DOI: 10.1172/JCI78558
Integrated compensatory network is activated in the absence of NCC phosphorylation
Abstract
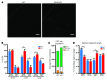
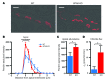
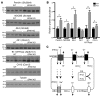
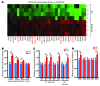
Thiazide diuretics are used to treat hypertension; however, compensatory processes in the kidney can limit antihypertensive responses to this class of drugs. Here, we evaluated compensatory pathways in SPAK kinase-deficient mice, which are unable to activate the thiazide-sensitive sodium chloride cotransporter NCC (encoded by Slc12a3). Global transcriptional profiling, combined with biochemical, cell biological, and physiological phenotyping, identified the gene expression signature of the response and revealed how it establishes an adaptive physiology. Salt reabsorption pathways were created by the coordinate induction of a multigene transport system, involving solute carriers (encoded by Slc26a4, Slc4a8, and Slc4a9), carbonic anhydrase isoforms, and V-type H⁺-ATPase subunits in pendrin-positive intercalated cells (PP-ICs) and ENaC subunits in principal cells (PCs). A distal nephron remodeling process and induction of jagged 1/NOTCH signaling, which expands the cortical connecting tubule with PCs and replaces acid-secreting α-ICs with PP-ICs, were partly responsible for the compensation. Salt reabsorption was also activated by induction of an α-ketoglutarate (α-KG) paracrine signaling system. Coordinate regulation of a multigene α-KG synthesis and transport pathway resulted in α-KG secretion into pro-urine, as the α-KG-activated GPCR (Oxgr1) increased on the PP-IC apical surface, allowing paracrine delivery of α-KG to stimulate salt transport. Identification of the integrated compensatory NaCl reabsorption mechanisms provides insight into thiazide diuretic efficacy.
Figures
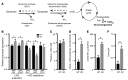
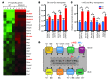
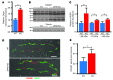

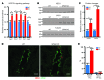
Comment in
-
Systems biology of diuretic resistance.J Clin Invest. 2015 May;125(5):1793-5. doi: 10.1172/JCI81505. Epub 2015 Apr 20. J Clin Invest. 2015. PMID: 25893597 Free PMC article.
Similar articles
-
Constitutively Active SPAK Causes Hyperkalemia by Activating NCC and Remodeling Distal Tubules.J Am Soc Nephrol. 2017 Sep;28(9):2597-2606. doi: 10.1681/ASN.2016090948. Epub 2017 Apr 25. J Am Soc Nephrol. 2017. PMID: 28442491 Free PMC article.
-
Systems biology of diuretic resistance.J Clin Invest. 2015 May;125(5):1793-5. doi: 10.1172/JCI81505. Epub 2015 Apr 20. J Clin Invest. 2015. PMID: 25893597 Free PMC article.
-
The Role of Epithelial Sodium Channel ENaC and the Apical Cl-/HCO3- Exchanger Pendrin in Compensatory Salt Reabsorption in the Setting of Na-Cl Cotransporter (NCC) Inactivation.PLoS One. 2016 Mar 10;11(3):e0150918. doi: 10.1371/journal.pone.0150918. eCollection 2016. PLoS One. 2016. PMID: 26963391 Free PMC article.
-
The interplay of renal potassium and sodium handling in blood pressure regulation: critical role of the WNK-SPAK-NCC pathway.J Hum Hypertens. 2019 Jul;33(7):508-523. doi: 10.1038/s41371-019-0170-6. Epub 2019 Feb 5. J Hum Hypertens. 2019. PMID: 30723251 Review.
-
α-Ketoglutarate drives electroneutral NaCl reabsorption in intercalated cells by activating a G-protein coupled receptor, Oxgr1.Curr Opin Nephrol Hypertens. 2017 Sep;26(5):426-433. doi: 10.1097/MNH.0000000000000353. Curr Opin Nephrol Hypertens. 2017. PMID: 28771454 Review.
Cited by
-
SPAK and OSR1 play essential roles in potassium homeostasis through actions on the distal convoluted tubule.J Physiol. 2016 Sep 1;594(17):4945-66. doi: 10.1113/JP272311. Epub 2016 May 29. J Physiol. 2016. PMID: 27068441 Free PMC article.
-
Constitutively Active SPAK Causes Hyperkalemia by Activating NCC and Remodeling Distal Tubules.J Am Soc Nephrol. 2017 Sep;28(9):2597-2606. doi: 10.1681/ASN.2016090948. Epub 2017 Apr 25. J Am Soc Nephrol. 2017. PMID: 28442491 Free PMC article.
-
Aldosterone: Renal Action and Physiological Effects.Compr Physiol. 2023 Mar 30;13(2):4409-4491. doi: 10.1002/cphy.c190043. Compr Physiol. 2023. PMID: 36994769 Free PMC article.
-
Renal and colonic potassium transporters in the pregnant rat.Am J Physiol Renal Physiol. 2018 Feb 1;314(2):F251-F259. doi: 10.1152/ajprenal.00288.2017. Epub 2017 Oct 18. Am J Physiol Renal Physiol. 2018. PMID: 29046297 Free PMC article.
-
Renal sodium and magnesium reabsorption are not coupled in a mouse model of Gordon syndrome.Physiol Rep. 2018 Jul;6(14):e13728. doi: 10.14814/phy2.13728. Physiol Rep. 2018. PMID: 30030908 Free PMC article.
References
-
- Ellison DH, Velazquez H, Wright FS. Thiazide-sensitive sodium chloride cotransport in early distal tubule. Am J Physiol. 1987;253(3 pt 2):F546–F554. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NR014129/NR/NINR NIH HHS/United States
- DK46493/DK/NIDDK NIH HHS/United States
- T32 HL072751/HL/NHLBI NIH HHS/United States
- T32DK07656/DK/NIDDK NIH HHS/United States
- R01 DK032839/DK/NIDDK NIH HHS/United States
- DK63049/DK/NIDDK NIH HHS/United States
- T32 DK007656/DK/NIDDK NIH HHS/United States
- GM074771/GM/NIGMS NIH HHS/United States
- R01 DK093501/DK/NIDDK NIH HHS/United States
- R01 DK046493/DK/NIDDK NIH HHS/United States
- R01 DK055881/DK/NIDDK NIH HHS/United States
- DK093501/DK/NIDDK NIH HHS/United States
- R01 DK063049/DK/NIDDK NIH HHS/United States
- DK55881/DK/NIDDK NIH HHS/United States
- 5T32HL072751/HL/NHLBI NIH HHS/United States
- P30NR014129/NR/NINR NIH HHS/United States
- R01 DK054231/DK/NIDDK NIH HHS/United States
- DK54231/DK/NIDDK NIH HHS/United States
- R01 GM074771/GM/NIGMS NIH HHS/United States
- R56 DK054231/DK/NIDDK NIH HHS/United States
- DK32839/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

