TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review
- PMID: 25893636
- PMCID: PMC4438204
- DOI: 10.1007/s10875-015-0152-6
TREC Based Newborn Screening for Severe Combined Immunodeficiency Disease: A Systematic Review
Abstract
Background: Newborn screening (NBS) by quantifying T cell receptor excision circles (TRECs) in neonatal dried blood spots (DBS) enables early diagnosis of severe combined immunodeficiency disease (SCID). In recent years, different screening algorithms for TREC based SCID screening were reported.
Purpose: To systematically review the diagnostic performance of published algorithms for TREC based NBS for SCID.
Methods: PubMed, EMBASE and the Cochrane Library were systematically searched for case series and prospective cohort studies describing TREC based NBS for SCID. We extracted TREC content and cut-off values, number of retests, repeat DBS and referrals, and type and number of typical SCID and other T cell lymphopenia (TCL) cases. We calculated positive predictive value (PPV), test sensitivity and SCID incidence.
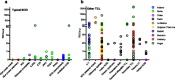
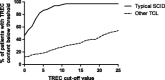
Results: Thirteen studies were included, re-confirming 89 known SCID cases in case series and reporting 53 new SCID cases in 3.15 million newborns. In case series, the sensitivity for typical SCID was 100%. In the prospective cohort studies, SCID incidence was ~1.7:100,000, re-test rate was 0.20-3.26%, repeat DBS rate 0.0-0.41% and referral rate 0.01-1.35%. PPV within the five largest cohorts was 0.8-11.2% for SCID and 18.3-81.0% for TCL. Individual TREC contents in all SCID patients was <25 TRECs/μl (except in those evaluated with the New York State assay).
Conclusions: The sensitivity of TREC based NBS for typical SCID was 100 %. The TREC cut-off score determines the percentage of non-SCID TCL cases detected in newborn screening for TCL. Adapting the screening algorithm for pre-term/ill infants reduces the amount of false positive test results.
Figures


References
-
- Dvorak CC, Cowan MJ, Logan BR, Notarangelo LD, Griffith LM, Puck JM, et al. The natural history of children with severe combined immunodeficiency: baseline features of the first fifty patients of the primary immune deficiency treatment consortium prospective study 6901. J Clin Immunol. 2013;33:1156–1164. doi: 10.1007/s10875-013-9917-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

