Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time
- PMID: 25893878
- PMCID: PMC4493546
- DOI: 10.3390/bios5020199
Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time
Abstract
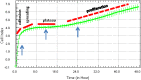
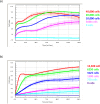
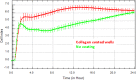
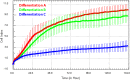
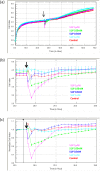
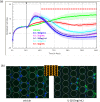
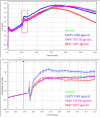
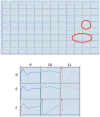
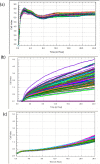
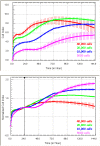
The xCELLigence technology is a real-time cellular biosensor, which measures the net adhesion of cells to high-density gold electrode arrays printed on custom-designed E-plates. The strength of cellular adhesion is influenced by a myriad of factors that include cell type, cell viability, growth, migration, spreading and proliferation. We therefore hypothesised that xCELLigence biosensor technology would provide a valuable platform for the measurement of drug responses in a multitude of different experimental, clinical or pharmacological contexts. In this manuscript, we demonstrate how xCELLigence technology has been invaluable in the identification of (1) not only if cells respond to a particular drug, but (2) the window of drug responsiveness. The latter aspect is often left to educated guess work in classical end-point assays, whereas biosensor technology reveals the temporal profile of the response in real time, which enables both acute responses and longer term responses to be profiled within the same assay. In our experience, the xCELLigence biosensor technology is suitable for highly targeted drug assessment and also low to medium throughput drug screening, which produces high content temporal data in real time.
Figures


References
-
- Van Oosterwijk J.G., van Ruler M.A., Briaire-de Bruijn I.H., Herpers B., Gelderblom H., van de Water B., Bovée J.V.M.G. Src kinases in chondrosarcoma chemoresistance and migration: Dasatinib sensitises to doxorubicin in TP53 mutant cells. Br. J. Cancer. 2013;109:1214–1222. doi: 10.1038/bjc.2013.451. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

