Resolving the contribution of the uncoupled phycobilisomes to cyanobacterial pulse-amplitude modulated (PAM) fluorometry signals
- PMID: 25893897
- PMCID: PMC4673099
- DOI: 10.1007/s11120-015-0141-x
Resolving the contribution of the uncoupled phycobilisomes to cyanobacterial pulse-amplitude modulated (PAM) fluorometry signals
Abstract
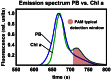
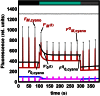
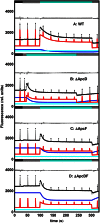
Pulse-amplitude modulated (PAM) fluorometry is extensively used to characterize photosynthetic organisms on the slow time-scale (1-1000 s). The saturation pulse method allows determination of the quantum yields of maximal (F(M)) and minimal fluorescence (F(0)), parameters related to the activity of the photosynthetic apparatus. Also, when the sample undergoes a certain light treatment during the measurement, the fluorescence quantum yields of the unquenched and the quenched states can be determined. In the case of cyanobacteria, however, the recorded fluorescence does not exclusively stem from the chlorophyll a in photosystem II (PSII). The phycobilins, the pigments of the cyanobacterial light-harvesting complexes, the phycobilisomes (PB), also contribute to the PAM signal, and therefore, F(0) and F(M) are no longer related to PSII only. We present a functional model that takes into account the presence of several fluorescent species whose concentrations can be resolved provided their fluorescence quantum yields are known. Data analysis of PAM measurements on in vivo cells of our model organism Synechocystis PCC6803 is discussed. Three different components are found necessary to fit the data: uncoupled PB (PB(free)), PB-PSII complexes, and free PSI. The free PSII contribution was negligible. The PB(free) contribution substantially increased in the mutants that lack the core terminal emitter subunits allophycocyanin D or allophycocyanin F. A positive correlation was found between the amount of PB(free) and the rate constants describing the binding of the activated orange carotenoid protein to PB, responsible for non-photochemical quenching.
Keywords: Cyanobacteria; Fluorescence quantum yield; Non-photochemical quenching; Phycobilisome; Pulse-amplitude modulated (PAM) fluorometry.
Figures

References
-
- Bryant DA. Cyanobacterial phycobilisomes: progress toward complete structural and functional analysis via molecular genetics. In: Bogorad L, Vasil IK, editors. The photosynthetic apparatus: molecular biology and operation. San Diego: Academic Press; 1991. pp. 257–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

