Clinical Features and Diagnostic Usefulness of Antibodies to Clustered Acetylcholine Receptors in the Diagnosis of Seronegative Myasthenia Gravis
- PMID: 25894002
- PMCID: PMC6044422
- DOI: 10.1001/jamaneurol.2015.0203
Clinical Features and Diagnostic Usefulness of Antibodies to Clustered Acetylcholine Receptors in the Diagnosis of Seronegative Myasthenia Gravis
Abstract
Importance: Cell-based assays (CBAs) were shown to improve detection of acetylcholine receptor (AChR) antibodies in patients with myasthenia gravis (MG). Herein, we asked whether these assays were able to help determine the diagnosis in patients studied in routine clinical practice.
Objectives: To determine the diagnostic usefulness of CBAs in the diagnosis of MG and to compare the clinical features of patients with antibodies only to clustered AChRs with those of patients with seronegative MG (SNMG).
Design, setting, and participants: All patients with clinical suspicion of MG who were seen within the Division of Clinical Neurology at the John Radcliffe Hospital in Oxford, England, between November 1, 2009, and November 30, 2013. Their serum antibodies and clinical features were studied.
Exposures: Radioimmunoprecipitation assay (RIPA) and CBA were used to test for standard AChR antibodies and antibodies to clustered AChRs in 138 patients. All available samples from patients with SNMG were retrospectively tested for lipoprotein receptor-related protein 4 (LRP4) antibodies.
Main outcomes and measures: Demographic, clinical, neurophysiological, and laboratory data.
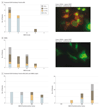
Results: In total, 138 patients were tested for antibodies to clustered AChRs, and 42 had a final diagnosis of MG. The clustered AChR CBA detected antibodies in 38.1% (16 of 42) of RIPA-negative patients with MG with 100% specificity. All patients with SNMG who were tested for LRP4 antibodies (21 of 26) were negative by CBA. Compared with patients with SNMG, patients with antibodies only to clustered AChRs had frequent prepubertal onset (62.5% [median age, 6 years; age range, 1-52 years] vs 11.5% [median age, 38 years; age range, 2-72 years], P ≤ .05), high prevalence of ocular MG (62.5% vs 42.3%), milder disease severity with less bulbar involvement (25.0% vs 46.2%), and absence of respiratory symptoms (0% vs 23.1%). Response to treatment and prognosis was good, with a reduced need for thymectomy (6.3% vs 19.2%) and a high proportion of patients going into remission (50.0% vs 8.3%, P ≤ .05). These observations also apply to the classic AChR MG phenotype seen in large series.
Conclusions and relevance: Cell-based assay is a useful procedure in the routine diagnosis of RIPA-negative MG, particularly in children. Patients with antibodies only to clustered AChRs appear to be younger and have milder disease than other patients with MG. These observations will have implications in planning treatment.
Conflict of interest statement
Figures
Comment in
-
Unraveling the Enigma of Seronegative Myasthenia Gravis.JAMA Neurol. 2015 Jun;72(6):630-1. doi: 10.1001/jamaneurol.2015.0205. JAMA Neurol. 2015. PMID: 25893727 No abstract available.
-
Neuromuscular disease: Improved diagnostic sensitivity can aid the correct choice of treatment for patients with myasthenia gravis.Nat Rev Neurol. 2015 Jun;11(6):308. doi: 10.1038/nrneurol.2015.78. Epub 2015 May 5. Nat Rev Neurol. 2015. PMID: 25939272 No abstract available.
References
-
- Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD. Antibody to acetylcholine receptor in myasthenia gravis: prevalence, clinical correlates, and diagnostic value. Neurology. 1976;26(11):1054–1059. - PubMed
-
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2(10):797–804. - PubMed
-
- Soliven BC, Lange DJ, Penn AS, et al. Seronegative myasthenia gravis. Neurology. 1988;38(4):514–517. - PubMed
-
- Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7(3):365–368. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

