Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo
- PMID: 25894291
- PMCID: PMC4429056
- DOI: 10.1038/nn.4001
Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo
Abstract
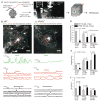
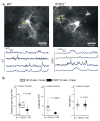
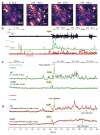
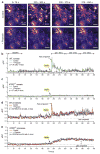
Intracellular Ca(2+) signaling is considered to be important for multiple astrocyte functions in neural circuits. However, mice devoid of inositol triphosphate type 2 receptors (IP3R2) reportedly lack all astrocyte Ca(2+) signaling, but display no neuronal or neurovascular deficits, implying that astrocyte Ca(2+) fluctuations are not involved in these functions. An assumption has been that the loss of somatic Ca(2+) fluctuations also reflects a similar loss in astrocyte processes. We tested this assumption and found diverse types of Ca(2+) fluctuations in astrocytes, with most occurring in processes rather than in somata. These fluctuations were preserved in Ip3r2(-/-) (also known as Itpr2(-/-)) mice in brain slices and in vivo, occurred in end feet, and were increased by G protein-coupled receptor activation and by startle-induced neuromodulatory responses. Our data reveal previously unknown Ca(2+) fluctuations in astrocytes and highlight limitations of studies that used Ip3r2(-/-) mice to evaluate astrocyte contributions to neural circuit function and mouse behavior.
Figures




References
-
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

