Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease
- PMID: 25894827
- PMCID: PMC4425550
- DOI: 10.1038/nm.3842
Homeostatic regulation of T cell trafficking by a B cell-derived peptide is impaired in autoimmune and chronic inflammatory disease
Abstract
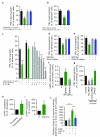
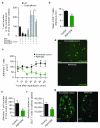
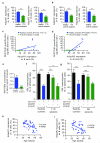
During an inflammatory response, lymphocyte recruitment into tissue must be tightly controlled because dysregulated trafficking contributes to the pathogenesis of chronic disease. Here we show that during inflammation and in response to adiponectin, B cells tonically inhibit T cell trafficking by secreting a peptide (PEPITEM) proteolytically derived from 14.3.3 zeta delta (14.3.3.ζδ) protein. PEPITEM binds cadherin-15 on endothelial cells, promoting synthesis and release of sphingosine-1 phosphate, which inhibits trafficking of T cells without affecting recruitment of other leukocytes. Expression of adiponectin receptors on B cells and adiponectin-induced PEPITEM secretion wanes with age, implying immune senescence of the pathway. Additionally, these changes are evident in individuals with type 1 diabetes or rheumatoid arthritis, and circulating PEPITEM in patient serum is reduced compared to that of healthy age-matched donors. In both diseases, tonic inhibition of T cell trafficking across inflamed endothelium is lost. Control of patient T cell trafficking is re-established by treatment with exogenous PEPITEM. Moreover, in animal models of peritonitis, hepatic ischemia-reperfusion injury, Salmonella infection, uveitis and Sjögren's syndrome, PEPITEM reduced T cell recruitment into inflamed tissues.
Figures



Comment in
-
A B cell-dependent mechanism restrains T cell transendothelial migration.Nat Med. 2015 May;21(5):424-6. doi: 10.1038/nm.3858. Nat Med. 2015. PMID: 25951526 Free PMC article.
-
T cell responses: B cells control T cell traffic.Nat Rev Immunol. 2015 Jun;15(6):332-3. doi: 10.1038/nri3860. Epub 2015 May 15. Nat Rev Immunol. 2015. PMID: 25976514 No abstract available.
-
[Regulation of T-cell trafficking in chronic inflammatory disease--far more complex!].Z Gastroenterol. 2015 Sep;53(9):1108. doi: 10.1055/s-0035-1553203. Epub 2015 Sep 14. Z Gastroenterol. 2015. PMID: 26367028 German. No abstract available.
References
METHODS-ONLY REFERENCES
-
- Rainger GE, Stone P, Morland CM, Nash GB. A novel system for investigating the ability of smooth muscle cells and fibroblasts to regulate adhesion of flowing leukocytes to endothelial cells. J Immunol Methods. 2001;255:73–82. - PubMed
-
- Cooke BM, Usami S, Perry I, Nash GB. A simplified method for culture of endothelial cells and analysis of adhesion of blood cells under conditions of flow. Microvasc Res. 1993;45:33–45. - PubMed
-
- Butler LM, Rainger GE, Rahman M, Nash GB. Prolonged culture of endothelial cells and deposition of basement membrane modify the recruitment of neutrophils. Exp Cell Res. 2005;310:22–32. - PubMed
-
- Eidhammer I, et al. Computational and Statistical Methods for Protein Quantification by Mass Spectrometry. John Wiley & Sons; New Jersey: 2012.
Publication types
MeSH terms
Substances
Grants and funding
- 19899/VAC_/Versus Arthritis/United Kingdom
- RG/12/7/29693/BHF_/British Heart Foundation/United Kingdom
- G0802382/MRC_/Medical Research Council/United Kingdom
- 18547/ARC_/Arthritis Research UK/United Kingdom
- BB/L009986/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- MR/K00414X/1/MRC_/Medical Research Council/United Kingdom
- MC_PC_12011/MRC_/Medical Research Council/United Kingdom
- 091709/WT_/Wellcome Trust/United Kingdom
- 19899/ARC_/Arthritis Research UK/United Kingdom
- 20088/ARC_/Arthritis Research UK/United Kingdom
- PG/11/49/28983/BHF_/British Heart Foundation/United Kingdom
- 19791/ARC_/Arthritis Research UK/United Kingdom
- CIC 12011/MRC_/Medical Research Council/United Kingdom
- ISSF 12/13-097825/Z/11/A/WT_/Wellcome Trust/United Kingdom
- PB-PG-0609-19093/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

