Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating mevalonate-isoprenoid pathway in vitro and in vivo
- PMID: 25895029
- PMCID: PMC4558155
- DOI: 10.18632/oncotarget.3684
Inhibition of insulin-like growth factor receptor/AKT/mammalian target of rapamycin axis targets colorectal cancer stem cells by attenuating mevalonate-isoprenoid pathway in vitro and in vivo
Abstract
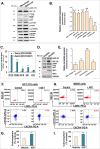
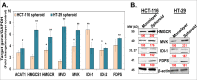
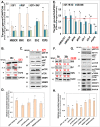
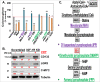
We observed a co-upregulation of the insulin-like growth factor receptor (IGF-1R)/AKT/mammalian target of rapamycin (mTOR) [InAT] axis and the mevalonate-isoprenoid biosynthesis (MIB) pathways in colorectal cancer stem cells (CSCs) in an unbiased approach. Hence, we hypothesized that the InAT axis might regulate the MIB pathway to govern colorectal CSCs growth. Stimulation (IGF-1) or inhibition (IGF-1R depletion and pharmacological inhibition of IGF-1R/mTOR) of the InAT axis produced induction or attenuation of CSC growth as well as expression of CSC markers and self-renewal factors respectively. Intriguingly, activation of the InAT axis (IGF-1) caused significant upregulation of the MIB pathway genes (both mRNA and protein); while its inhibition produced the opposite effects in colonospheres. More importantly, supplementation with dimethylallyl- and farnesyl-PP, MIB metabolites downstream of isopentenyl-diphosphate delta isomerase (IDI), but not mevalonate and isopentenyl-pp that are upstream of IDI, resulted in a near-complete reversal of the suppressive effect of the InAT axis inhibitors on CSCs growth. The latter findings suggest a specific regulation of the MIB pathway by the InAT axis distal to the target of statins that inhibit 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR). Effects of IGF-1R inhibition on colonic CSCs proliferation and the MIB pathway were confirmed in an 'in vivo' HCT-116 xenograft model. These observations establish a novel mechanistic link between the InAT axis that is commonly deregulated in colorectal cancer and the MIB pathway in regulation of colonic CSCs growth. Hence, the InAT-MIB corridor is a novel target for developing paradigm shifting optimum anti-CSCs therapies for colorectal cancer.
Keywords: cancer stem cells; insulin-like growth factor-1 receptor; isoprenoids; mTOR; mevalonate pathway.
Conflict of interest statement
The authors declare no competing financial or intellectual property interest.
Figures


References
-
- Gangemi R, Paleari L, Orengo AM, Cesario A, Chessa L, Ferrini S, Russo P. Cancer stem cells: a new paradigm for understanding tumor growth and progression and drug resistance. Curr Med Chem. 2009;16:1688–1703. - PubMed
-
- Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. - PubMed
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Vaiopoulos AG, Kostakis ID, Koutsilieris M, Papavassiliou AG. Colorectal cancer stem cells. Stem Cells. 2012;30:363–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

