Ginkgolic acid suppresses the development of pancreatic cancer by inhibiting pathways driving lipogenesis
- PMID: 25895130
- PMCID: PMC4673245
- DOI: 10.18632/oncotarget.3663
Ginkgolic acid suppresses the development of pancreatic cancer by inhibiting pathways driving lipogenesis
Abstract
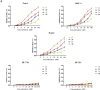
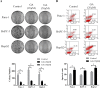
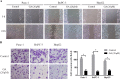
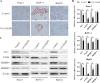
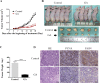
Ginkgolic acid (GA) is a botanical drug extracted from the seed coat of Ginkgo biloba L. with a wide range of bioactive properties, including anti-tumor effect. However, whether GA has antitumor effect on pancreatic cancer cells and the underlying mechanisms have yet to be investigated. In this study, we show that GA suppressed the viability of cancer cells but has little toxicity on normal cells, e.g, HUVEC cells. Furthermore, treatment of GA resulted in impaired colony formation, migration, and invasion ability and increased apoptosis of cancer cells. In addition, GA inhibited the de novo lipogenesis of cancer cells through inducing activation of AMP-activated protein kinase (AMPK) signaling and downregulated the expression of key enzymes (e.g. acetyl-CoA carboxylase [ACC], fatty acid synthase [FASN]) involved in lipogenesis. Moreover, the in vivo experiment showed that GA reduced the expression of the key enzymes involved in lipogenesis and restrained the tumor growth. Taken together, our results suggest that GA may serve as a new candidate against tumor growth of pancreatic cancer partially through targeting pathway driving lipogenesis.
Keywords: AMP-activated protein kinase (AMPK); cancer metabolism; ginkgolic acid (GA); lipogenesis; pancreatic cancer.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures

References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. - PubMed
-
- Skill NJ, Scott RE, Wu J, Maluccio MA. Hepatocellular carcinoma associated lipid metabolism reprogramming. J Surg Res. 2011;169:51–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

