The BALB/c mouse: Effect of standard vivarium lighting on retinal pathology during aging
- PMID: 25895728
- PMCID: PMC4446204
- DOI: 10.1016/j.exer.2015.04.009
The BALB/c mouse: Effect of standard vivarium lighting on retinal pathology during aging
Abstract
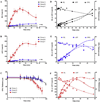
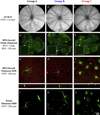
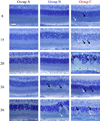
BALB/cJ mice housed under normal vivarium lighting conditions can exhibit profound retinal abnormalities, including retinal infoldings, autofluorescent inflammatory cells, and photoreceptor degeneration. To explore the sensitivity of the outer retina to cyclic lighting during aging, a cohort of BALB/cJ mice was evaluated with Scanning Laser Ophthalmoscopy (SLO), Spectral-Domain Optical Coherence Tomography (OCT) and conventional histopathology. Mice were bred and reared in a low-illuminance (extracage/intracage: 13 lx/1 lx) vivarium under cyclic light (14 h light: 10 h dark). Retinal imaging (around postnatal day 70) was performed to screen for any pre-existing abnormalities and to establish a baseline. Mice with normal retinas were separated into groups (A, B, C) and placed on bottom (Groups A & B) or top (Group C) of the cage racks where cage illumination was <10 & 150 lx respectively. Experimental groups B & C were imaged multiple times over a 17 month period. Mice from group A (controls) were imaged only once post-baseline at various times for comparison to groups B & C. Mice were assessed by histology at 8, 15, 20, 36, and 56 weeks and immunohistochemistry at 15 weeks post-baseline. SLO and OCT retinal images were measured and the resulting trends displayed as a function of age and light exposure. Retinal lesions (RL) and autofluorescent foci (AFF) were identified with histology as photoreceptor layer infoldings (IF) and localized microglia/macrophages (MM), respectively. Few RL and AFF were evident at baseline. Retinal infoldings were the earliest changes followed by subjacent punctate autofluorescent MM. The colocalization of IF and MM suggests a causal relationship. The incidence of these pathological features increased in all groups relative to baseline. OCT imaging revealed thinning of the outer nuclear layer (ONL) in all groups at 1 year relative to baseline. ONL thinning followed an exponential rate of change but the decay constant varied depending on intensity of illumination of the groups. Advanced age and top row illuminance conditions resulted in significant photoreceptor cell loss as judged by decreased thickness of the ONL. Photoreceptor loss was preceded by both retinal infoldings and the presence of autofluorescent inflammatory cells in the outer retina, suggesting that these changes are early indicators of light toxicity in the BALB/cJ mouse.
Keywords: BALB/c; Degeneration; Imaging; Inflammation; Infolding; Mice; Phototoxicity; Retina.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures




References
-
- Akhmedov NB, Piriev NI, Chang B, Rapoport AL, Hawes NL, Nishina PM, Nusinowitz S, Heckenlively JR, Roderick TH, Kozak CA, Danciger M, Davisson MT, Farber DB. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. U. S.A. 2000;97:5551–5556. - PMC - PubMed
-
- Bell BA, Kaul C, Rayborn ME, Hollyfield JG. Baseline imaging reveals preexisting retinal abnormalities in mice. Adv. Exp. Med. Biol. 2012;723:459–469. - PubMed
-
- Cruz-Guilloty F, Saeed AM, Echegaray JJ, Duffort S, Ballmick A, Tan Y, Betancourt M, Viteri E, Ramkhellawan GC, Ewald E, Feuer W, Huang D, Wen R, Hong L, Wang H, Laird JM, Sene A, Apte RS, Salomon RG, Hollyfield JG, Perez VL. Infiltration of proinflammatory m1 macrophages into the outer retina precedes damage in a mouse model of age-related macular degeneration. Int. J. Inflam. 2013;2013:503725. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

