Theranostic Magnetic Nanostructures (MNS) for Cancer
- PMID: 25895864
- PMCID: PMC4494108
- DOI: 10.1007/978-3-319-16555-4_3
Theranostic Magnetic Nanostructures (MNS) for Cancer
Abstract
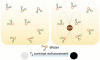
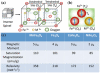
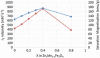
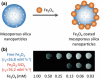
Despite the complexities of cancer, remarkable diagnostic and therapeutic advances have been made during the past decade, which include improved genetic, molecular, and nanoscale understanding of the disease. Physical science and engineering, and nanotechnology in particular, have contributed to these developments through out-of-the-box ideas and initiatives from perspectives that are far removed from classical biological and medicinal aspects of cancer. Nanostructures, in particular, are being effectively utilized in sensing/diagnostics of cancer while nanoscale carriers are able to deliver therapeutic cargo for timed and controlled release at localized tumor sites. Magnetic nanostructures (MNS) have especially attracted considerable attention of researchers to address cancer diagnostics and therapy. A significant part of the promise of MNS lies in their potential for "theranostic" applications, wherein diagnostics makes use of the enhanced localized contrast in magnetic resonance imaging (MRI) while therapy leverages the ability of MNS to heat under external radio frequency (RF) field for thermal therapy or use of thermal activation for release of therapy cargo. In this chapter, we report some of the key developments in recent years in regard to MNS as potential theranostic carriers. We describe that the r₂relaxivity of MNS can be maximized by allowing water (proton) diffusion in the vicinity of MNS by polyethylene glycol (PEG) anchoring, which also facilitates excellent fluidic stability in various media and extended in vivo circulation while maintaining high r₂values needed for T₂-weighted MRI contrast. Further, the specific absorption rate (SAR) required for thermal activation of MNS can be tailored by controlling composition and size of MNS. Together, emerging MNS show considerable promise to realize theranostic potential. We discuss that properly functionalized MNS can be designed to provide remarkable in vivo stability and accompanying pharmacokinetics exhibit organ localization that can be tailored for specific applications. In this context, even iron-based MNS show extended circulation as well as diverse organ accumulation beyond liver, which otherwise renders MNS potentially toxic to liver function. We believe that MNS, including those based on iron oxides, have entered a renaissance era where intelligent synthesis, functionalization, stabilization, and targeting provide ample evidence for applications in localized cancer theranostics.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

