Gas-Phase Anionic σ-Adduct (Trans)formations in Heteroaromatic Systems
- PMID: 25895890
- PMCID: PMC4475249
- DOI: 10.1007/s13361-015-1122-1
Gas-Phase Anionic σ-Adduct (Trans)formations in Heteroaromatic Systems
Abstract
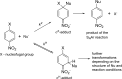
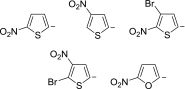
Anions of nitroderivatives of thiophene and furan were subjected to the reactions with selected C-H acids in the gas phase. Various structures and reaction pathways were proposed for the observed ionic products. In general, the reactions of heteroaromatic anions with C-H acids may be divided into three groups, depending on the proton affinity difference between C-H acid's conjugate base and heteroaromatic anion (ΔPA). The proton transfer from C-H acid to heteroaromatic anion is a dominant process in the reactions for which ΔPA < 0 kcal mol(-1), whereas the reactions with high ΔPA (ΔPA > 16 kcal mol(-1)) do not lead to any ionic products. The formation of σ-adducts and products of their further transformations according to the VNS, SNAr, cine, and tele substitution mechanisms have been proposed for reactions with moderate ΔPA. The other possible mechanisms as SN2 reaction, nucleophilic addition to the cyano group, ring-opening pathway, and halogenophilic reaction have also been discussed to contribute in the reactions between heteroaromatic anions and C-H acids.
Figures


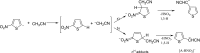



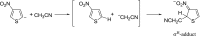
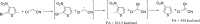
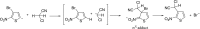
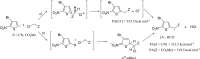
Similar articles
-
Negative ion gas-phase chemistry of arenes.Mass Spectrom Rev. 2016 Jan-Feb;35(1):123-46. doi: 10.1002/mas.21467. Epub 2015 Apr 6. Mass Spectrom Rev. 2016. PMID: 25851641 Review.
-
Generation and reactions of anionic sigma-adducts of 1,3-dinitrobenzene and 1,3,5-trinitrobenzene with carbanions in a gas phase, using an electrospray ion source as the chemical reactor.J Am Soc Mass Spectrom. 2004 Jun;15(6):927-33. doi: 10.1016/j.jasms.2004.03.012. J Am Soc Mass Spectrom. 2004. PMID: 15144984
-
Gas phase reactions of 1,3,5-triazine: proton transfer, hydride transfer, and anionic σ-adduct formation.J Am Soc Mass Spectrom. 2011 Jul;22(7):1260-72. doi: 10.1007/s13361-011-0133-9. Epub 2011 Apr 19. J Am Soc Mass Spectrom. 2011. PMID: 21953109
-
Aromatic nucleophilic substitution (SNAr) reactions of 1,2- and 1,4-halonitrobenzenes and 1,4-dinitrobenzene with carbanions in the gas phase.J Am Soc Mass Spectrom. 2007 Aug;18(8):1351-63. doi: 10.1016/j.jasms.2007.04.005. Epub 2007 Apr 25. J Am Soc Mass Spectrom. 2007. PMID: 17555982
-
Formation and Reactions of Brønsted and Lewis Acid Adducts with Electron-Rich Heteroaromatic Compounds.Molecules. 2024 Jul 2;29(13):3151. doi: 10.3390/molecules29133151. Molecules. 2024. PMID: 38999101 Free PMC article. Review.
References
-
- Terrier, F.: Modern Nucleophilic Aromatic Substitution. Wiley-VCH, Weinheim 1-464 (2013)
-
- Buncel E, Dust JM, Terrier F. Rationalizing the regioselectivity in polynitroarene anionic σ-adduct formation. Relevance to nucleophilic aromatic substitution. Chem. Rev. 1995;95:2261–2280. doi: 10.1021/cr00039a001. - DOI
-
- Mąkosza M. Nucleophilic substitution of hydrogen in nitroarenes: a new chapter of aromatic chemistry. Synthesis. 2011;2011:2341–2356. doi: 10.1055/s-0030-1260668. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

