Living in the matrix: assembly and control of Vibrio cholerae biofilms
- PMID: 25895940
- PMCID: PMC4437738
- DOI: 10.1038/nrmicro3433
Living in the matrix: assembly and control of Vibrio cholerae biofilms
Abstract
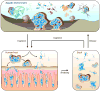
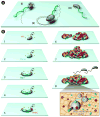
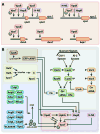
Nearly all bacteria form biofilms as a strategy for survival and persistence. Biofilms are associated with biotic and abiotic surfaces and are composed of aggregates of cells that are encased by a self-produced or acquired extracellular matrix. Vibrio cholerae has been studied as a model organism for understanding biofilm formation in environmental pathogens, as it spends much of its life cycle outside of the human host in the aquatic environment. Given the important role of biofilm formation in the V. cholerae life cycle, the molecular mechanisms underlying this process and the signals that trigger biofilm assembly or dispersal have been areas of intense investigation over the past 20 years. In this Review, we discuss V. cholerae surface attachment, various matrix components and the regulatory networks controlling biofilm formation.
Figures


Similar articles
-
Host intestinal signal-promoted biofilm dispersal induces Vibrio cholerae colonization.Infect Immun. 2015 Jan;83(1):317-23. doi: 10.1128/IAI.02617-14. Epub 2014 Nov 3. Infect Immun. 2015. PMID: 25368110 Free PMC article.
-
Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation.Mol Microbiol. 2011 Nov;82(4):1015-37. doi: 10.1111/j.1365-2958.2011.07867.x. Epub 2011 Oct 27. Mol Microbiol. 2011. PMID: 22032623 Free PMC article.
-
Outer membrane vesicles and the outer membrane protein OmpU govern Vibrio cholerae biofilm matrix assembly.mBio. 2024 Feb 14;15(2):e0330423. doi: 10.1128/mbio.03304-23. Epub 2024 Jan 11. mBio. 2024. PMID: 38206049 Free PMC article.
-
Mechanisms Underlying Vibrio cholerae Biofilm Formation and Dispersion.Annu Rev Microbiol. 2022 Sep 8;76:503-532. doi: 10.1146/annurev-micro-111021-053553. Epub 2022 Jun 7. Annu Rev Microbiol. 2022. PMID: 35671532 Review.
-
Vibrio cholerae Biofilms and Cholera Pathogenesis.PLoS Negl Trop Dis. 2016 Feb 4;10(2):e0004330. doi: 10.1371/journal.pntd.0004330. eCollection 2016 Feb. PLoS Negl Trop Dis. 2016. PMID: 26845681 Free PMC article. Review.
Cited by
-
Quorum-sensing control of matrix protein production drives fractal wrinkling and interfacial localization of Vibrio cholerae pellicles.Nat Commun. 2022 Oct 13;13(1):6063. doi: 10.1038/s41467-022-33816-6. Nat Commun. 2022. PMID: 36229546 Free PMC article.
-
Metabolomic Profiling of the Responses of Planktonic and Biofilm Vibrio cholerae to Silver Nanoparticles.Antibiotics (Basel). 2022 Nov 2;11(11):1534. doi: 10.3390/antibiotics11111534. Antibiotics (Basel). 2022. PMID: 36358189 Free PMC article.
-
Sustained sensing as an emerging principle in second messenger signaling systems.Curr Opin Microbiol. 2016 Dec;34:119-126. doi: 10.1016/j.mib.2016.08.010. Epub 2016 Oct 1. Curr Opin Microbiol. 2016. PMID: 27700990 Free PMC article. Review.
-
Importance of the biofilm matrix for the erosion stability of Bacillus subtilis NCIB 3610 biofilms.RSC Adv. 2019 Apr 11;9(20):11521-11529. doi: 10.1039/c9ra01955c. eCollection 2019 Apr 9. RSC Adv. 2019. PMID: 35520264 Free PMC article.
-
Secreted Proteases Control the Timing of Aggregative Community Formation in Vibrio cholerae.mBio. 2021 Dec 21;12(6):e0151821. doi: 10.1128/mBio.01518-21. Epub 2021 Nov 23. mBio. 2021. PMID: 34809464 Free PMC article.
References
-
- Charles RC, Ryan ET. Cholera in the 21st century. Curr Opin Infect Dis. 2011;24:472–7. - PubMed
-
- Islam MS, et al. Biofilm acts as a microenvironment for plankton-associated Vibrio cholerae in the aquatic environment of Bangladesh. Microbiol Immunol. 2007;51:369–79. - PubMed
-
- Faruque SM, et al. Transmissibility of cholera: in vivo-formed biofilms and their relationship to infectivity and persistence in the environment. Proc Natl Acad Sci U S A. 2006;103:6350–5. This study demonstrates that the ingestion of metabolically quiescent V. cholerae cells can lead to the formation of in vivo biofilms that are hyperinfective when excreted in patient stool. - PMC - PubMed
-
- Alam M, et al. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci U S A. 2007;104:17801–6. This study reveals the crucial role of biofilms in maintaining environmental reservoirs between epidemics, as well as the role of host passage in activation of metabolically quiescent cells. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

