The Serine Protease Autotransporter Pic Modulates Citrobacter rodentium Pathogenesis and Its Innate Recognition by the Host
- PMID: 25895966
- PMCID: PMC4468532
- DOI: 10.1128/IAI.00025-15
The Serine Protease Autotransporter Pic Modulates Citrobacter rodentium Pathogenesis and Its Innate Recognition by the Host
Abstract
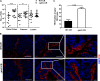
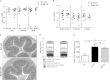
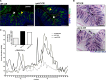
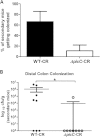
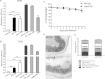
Bacterial pathogens produce a number of autotransporters that possess diverse functions. These include the family of serine protease autotransporters of Enterobacteriaceae (SPATEs) produced by enteric pathogens such as Shigella flexneri and enteroaggregative Escherichia coli. Of these SPATEs, one termed "protein involved in colonization," or Pic, has been shown to possess mucinase activity in vitro, but to date, its role in in vivo enteric pathogenesis is unknown. Testing a pic null (ΔpicC) mutant in Citrobacter rodentium, a natural mouse pathogen, found that the C. rodentium ΔpicC strain was impaired in its ability to degrade mucin in vitro compared to the wild type. Upon infection of mice, the ΔpicC mutant exhibited a hypervirulent phenotype with dramatically heavier pathogen burdens found in intestinal crypts. ΔpicC mutant-infected mice suffered greater barrier disruption and more severe colitis and weight loss, necessitating their euthanization between 10 and 14 days postinfection. Notably, the virulence of the ΔpicC mutant was normalized when the picC gene was restored; however, a PicC point mutant causing loss of mucinase activity did not replicate the ΔpicC phenotype. Exploring other aspects of PicC function, the ΔpicC mutant was found to aggregate to higher levels in vivo than wild-type C. rodentium. Moreover, unlike the wild type, the C. rodentium ΔpicC mutant had a red, dry, and rough (RDAR) morphology in vitro and showed increased activation of the innate receptor Toll-like receptor 2 (TLR2). Interestingly, the C. rodentium ΔpicC mutant caused a degree of pathology similar to that of wild-type C. rodentium when infecting TLR2-deficient mice, showing that despite its mucinase activity, PicC's major role in vivo may be to limit C. rodentium's stimulation of the host's innate immune system.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

