Abolishing Cell Wall Glycosylphosphatidylinositol-Anchored Proteins in Candida albicans Enhances Recognition by Host Dectin-1
- PMID: 25895969
- PMCID: PMC4468527
- DOI: 10.1128/IAI.00097-15
Abolishing Cell Wall Glycosylphosphatidylinositol-Anchored Proteins in Candida albicans Enhances Recognition by Host Dectin-1
Abstract
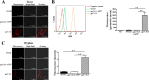
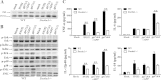
Fungi can shield surface pathogen-associated molecular patterns (PAMPs) for evading host immune attack. The most common and opportunistic human pathogen, Candida albicans, can shield β-(1 3)-glucan on the cell wall, one of the major PAMPs, to avoid host phagocyte Dectin-1 recognition. The way to interfere in the shielding process for more effective antifungal defense is not well established. In this study, we found that deletion of the C. albicans GPI7 gene, which was responsible for adding ethanolaminephosphate to the second mannose in glycosylphosphatidylinositol (GPI) biosynthesis, could block the attachment of most GPI-anchored cell wall proteins (GPI-CWPs) to the cell wall and subsequently unmask the concealed β-(1,3)-glucan. Neutrophils could kill the uncloaked gpi7 mutant more efficiently with an augmented respiratory burst. The gpi7 mutant also stimulated Dectin-1-dependent immune responses of macrophages, including activation of nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways and secretion of specific cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IL-12p40. Furthermore, the gpi7 null mutant could induce an enhanced inflammatory response through promoting significant recruitment of neutrophils and monocytes and could stimulate stronger Th1 and Th17 cell responses to fungal infections in vivo. These in vivo phenotypes also were Dectin-1 dependent. Thus, we assume that GPI-CWPs are involved in the immune mechanism of C. albicans escaping from host recognition by Dectin-1. Our studies also indicate that the blockage of GPI anchor synthesis is a strategy to inhibit C. albicans evading host recognition.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures





Similar articles
-
Mnn10 Maintains Pathogenicity in Candida albicans by Extending α-1,6-Mannose Backbone to Evade Host Dectin-1 Mediated Antifungal Immunity.PLoS Pathog. 2016 May 4;12(5):e1005617. doi: 10.1371/journal.ppat.1005617. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27144456 Free PMC article.
-
Enhanced proinflammatory response to the Candida albicans gpi7 null mutant by murine cells.Microbes Infect. 2008 Apr;10(4):382-9. doi: 10.1016/j.micinf.2007.12.018. Epub 2008 Jan 9. Microbes Infect. 2008. PMID: 18403244
-
Complete glycosylphosphatidylinositol anchors are required in Candida albicans for full morphogenesis, virulence and resistance to macrophages.Mol Microbiol. 2002 May;44(3):841-53. doi: 10.1046/j.1365-2958.2002.02926.x. Mol Microbiol. 2002. PMID: 11994163
-
Face/Off: The Interchangeable Side of Candida Albicans.Front Cell Infect Microbiol. 2020 Jan 28;9:471. doi: 10.3389/fcimb.2019.00471. eCollection 2019. Front Cell Infect Microbiol. 2020. PMID: 32047726 Free PMC article. Review.
-
[Contribution of dectin-1 to the recognition of fungal cell wall products and the activation of innate immune response].Nihon Ishinkin Gakkai Zasshi. 2006;47(3):185-94. doi: 10.3314/jjmm.47.185. Nihon Ishinkin Gakkai Zasshi. 2006. PMID: 16940953 Review. Japanese.
Cited by
-
Comparative Analysis of Secretomes from Ectomycorrhizal Fungi with an Emphasis on Small-Secreted Proteins.Front Microbiol. 2015 Nov 18;6:1278. doi: 10.3389/fmicb.2015.01278. eCollection 2015. Front Microbiol. 2015. PMID: 26635749 Free PMC article.
-
Genetic Screening of Candida albicans Inactivation Mutants Identifies New Genes Involved in Macrophage-Fungal Cell Interactions.Front Microbiol. 2022 Apr 5;13:833655. doi: 10.3389/fmicb.2022.833655. eCollection 2022. Front Microbiol. 2022. PMID: 35450285 Free PMC article.
-
The secretory Candida effector Sce1 licenses fungal virulence by masking the immunogenic β-1,3-glucan and promoting apoptosis of the host cells.mLife. 2023 Jun 26;2(2):159-177. doi: 10.1002/mlf2.12066. eCollection 2023 Jun. mLife. 2023. PMID: 38817625 Free PMC article.
-
Caspofungin-induced β(1,3)-glucan exposure in Candida albicans is driven by increased chitin levels.mBio. 2023 Aug 31;14(4):e0007423. doi: 10.1128/mbio.00074-23. Epub 2023 Jun 28. mBio. 2023. PMID: 37377417 Free PMC article.
-
Cek1 regulates ß(1,3)-glucan exposure through calcineurin effectors in Candida albicans.PLoS Genet. 2022 Sep 19;18(9):e1010405. doi: 10.1371/journal.pgen.1010405. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36121853 Free PMC article.
References
-
- Hsu FC, Lin PC, Chi CY, Ho MW, Ho CM, Wang JH. 2009. Prognostic factors for patients with culture-positive Candida infection undergoing abdominal surgery. J Microbiol Immunol Infect 42:378–384. - PubMed
-
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48:1695–1703. doi:10.1086/599039. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

