AP-1 Transcription Factor Serves as a Molecular Switch between Chlamydia pneumoniae Replication and Persistence
- PMID: 25895972
- PMCID: PMC4468534
- DOI: 10.1128/IAI.03083-14
AP-1 Transcription Factor Serves as a Molecular Switch between Chlamydia pneumoniae Replication and Persistence
Abstract
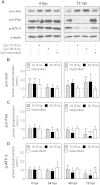
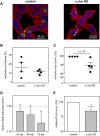
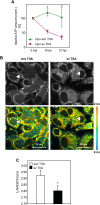
Chlamydia pneumoniae is a Gram-negative bacterium that causes acute or chronic respiratory infections. As obligate intracellular pathogens, chlamydiae efficiently manipulate host cell processes to ensure their intracellular development. Here we focused on the interaction of chlamydiae with the host cell transcription factor activator protein 1 (AP-1) and its consequence on chlamydial development. During Chlamydia pneumoniae infection, the expression and activity of AP-1 family proteins c-Jun, c-Fos, and ATF-2 were regulated in a time- and dose-dependent manner. We observed that the c-Jun protein and its phosphorylation level significantly increased during C. pneumoniae development. Small interfering RNA knockdown of the c-Jun protein in HEp-2 cells reduced the chlamydial load, resulting in smaller inclusions and significantly lower chlamydial recovery. Furthermore, inhibition of the c-Jun-containing AP-1 complexes using tanshinone IIA changed the replicative infection phenotype into a persistent one. Tanshinone IIA-dependent persistence was characterized by smaller, aberrant inclusions, a strong decrease in the chlamydial load, and significantly reduced chlamydial recovery, as well as by the reversibility of the reduced recovery after the removal of tanshinone IIA. Interestingly, not only was tanshinone IIA treatment accompanied by a significant decrease of ATP levels, but fluorescence live cell imaging analysis by two-photon microscopy revealed that tanshinone IIA treatment also resulted in a decreased fluorescence lifetime of protein-bound NAD(P)H inside the chlamydial inclusion, indicating that chlamydial reticulate bodies have decreased metabolic activity. In all, these data demonstrate that the AP-1 transcription factor is involved in C. pneumoniae development, with tanshinone IIA treatment resulting in persistence.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Chlamydia pneumoniae expresses genes required for DNA replication but not cytokinesis during persistent infection of HEp-2 cells.Infect Immun. 2001 Sep;69(9):5423-9. doi: 10.1128/IAI.69.9.5423-5429.2001. Infect Immun. 2001. PMID: 11500413 Free PMC article.
-
Fluorescence lifetime imaging unravels C. trachomatis metabolism and its crosstalk with the host cell.PLoS Pathog. 2011 Jul;7(7):e1002108. doi: 10.1371/journal.ppat.1002108. Epub 2011 Jul 14. PLoS Pathog. 2011. PMID: 21779161 Free PMC article.
-
Inhibition of tRNA Synthetases Induces Persistence in Chlamydia.Infect Immun. 2020 Mar 23;88(4):e00943-19. doi: 10.1128/IAI.00943-19. Print 2020 Mar 23. Infect Immun. 2020. PMID: 31964747 Free PMC article.
-
[Chlamydia pneumoniae infections].Kekkaku. 2006 Sep;81(9):581-8. Kekkaku. 2006. PMID: 17037392 Review. Japanese.
-
Imaging of Chlamydia and host cell metabolism.Future Microbiol. 2014;9(4):509-21. doi: 10.2217/fmb.14.13. Future Microbiol. 2014. PMID: 24810350 Review.
Cited by
-
Associations of cold-inducible RNA-binding protein with bacterial load, proinflammatory cytokines and mortality from pneumonia.Clin Transl Sci. 2024 Jun;17(6):e13850. doi: 10.1111/cts.13850. Clin Transl Sci. 2024. PMID: 38807464 Free PMC article.
-
Arthropod transcriptional activator protein-1 (AP-1) aids tick-rickettsial pathogen survival in the cold.Sci Rep. 2018 Jul 30;8(1):11409. doi: 10.1038/s41598-018-29654-6. Sci Rep. 2018. PMID: 30061607 Free PMC article.
-
Research Progress on Chemical Compositions, Pharmacological Activities, and Toxicities of Quinone Compounds in Traditional Chinese Medicines.Toxics. 2025 Jun 30;13(7):559. doi: 10.3390/toxics13070559. Toxics. 2025. PMID: 40711004 Free PMC article. Review.
-
Chlamydia trachomatis induces the transcriptional activity of host YAP in a Hippo-independent fashion.Front Cell Infect Microbiol. 2023 Feb 27;13:1098420. doi: 10.3389/fcimb.2023.1098420. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 36923592 Free PMC article.
-
Pleiotropic roles of metallothioneins as regulators of chondrocyte apoptosis and catabolic and anabolic pathways during osteoarthritis pathogenesis.Ann Rheum Dis. 2016 Nov;75(11):2045-2052. doi: 10.1136/annrheumdis-2015-208406. Epub 2016 Feb 22. Ann Rheum Dis. 2016. PMID: 26903440 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

