Construction and characterization of a sox9b transgenic reporter line
- PMID: 25896205
- PMCID: PMC4510993
- DOI: 10.1387/ijdb.140288jp
Construction and characterization of a sox9b transgenic reporter line
Abstract
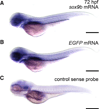
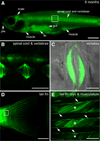
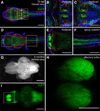
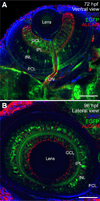
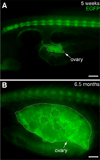
The transcription factor SOX9 is a member of the SRY-related high-mobility-group box (SOX) superfamily of genes. In mammals, Sox9 plays important roles in many developmental processes including craniofacial, skeletal and heart morphogenesis, retinal and brain development, and gonad differentiation. Human mutations in SOX9 or the SOX9 promoter result in campomelic dysplasia, a severe genetic disorder, which disrupts skeletal, craniofacial, cardiac, neural and reproductive development. Due to the duplication of the teleost fish genome, zebrafish (Danio rerio) have two Sox9 genes: sox9a and sox9b. Loss of sox9b in zebrafish results in loss of function phenotypes that are similar to those observed in humans and mice. In order to generate a transgenic sox9b:EGFP reporter line, we cloned a 2450 bp fragment of the sox9b promoter and fused it to an EGFP reporter. Consistent with reported sox9b expression and function, we observed sox9b:EGFP in the developing heart, skeletal and craniofacial structures, brain, retina, and ovaries. Our resulting transgenic line is a useful tool for identifying and studying sox9b function in development and visualizing a number of zebrafish organs and tissues in which sox9b is normally expressed.
Figures





References
-
- Chiang EFL, Pai CI, Wyatt M, Yan YL, Postlethwait J, Chung BC. Two sox9 genes on duplicated zebrafish chromosomes: Expression of similar transcription activators in distinct sites. Dev Bio. 2001;231:149–163. - PubMed
-
- Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, Brook JD, Schafer AJ. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. - PubMed
-
- Houston CS, Opitz JM, Spranger JW, Macpherson RI, Reed MH, GilBert EF, Herrmann J, Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al., in 1971. Am J Med Genet. 1983;15:3–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

