Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor
- PMID: 25896248
- PMCID: PMC4817886
- DOI: 10.1038/mt.2015.60
Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor
Abstract
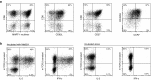
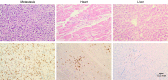
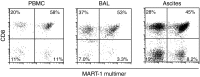
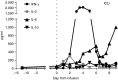
Here, we describe a fatal serious adverse event observed in a patient infused with autologous T-cell receptor (TCR) transduced T cells. This TCR, originally obtained from a melanoma patient, recognizes the well-described HLA-A*0201 restricted 26-35 epitope of MART-1, and was not affinity enhanced. Patient 1 with metastatic melanoma experienced a cerebral hemorrhage, epileptic seizures, and a witnessed cardiac arrest 6 days after cell infusion. Three days later, the patient died from multiple organ failure and irreversible neurologic damage. After T-cell infusion, levels of IL-6, IFN-γ, C-reactive protein (CRP), and procalcitonin increased to extreme levels, indicative of a cytokine release syndrome or T-cell-mediated inflammatory response. Infused T cells could be recovered from blood, broncho-alveolar lavage, ascites, and after autopsy from tumor sites and heart tissue. High levels of NT-proBNP indicate semi-acute heart failure. No cross reactivity of the modified T cells toward a beating cardiomyocyte culture was observed. Together, these observations suggest that high levels of inflammatory cytokines alone or in combination with semi-acute heart failure and epileptic seizure may have contributed substantially to the occurrence of the acute and lethal event. Protocol modifications to limit the risk of T-cell activation-induced toxicity are discussed.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

