USP18 lack in microglia causes destructive interferonopathy of the mouse brain
- PMID: 25896511
- PMCID: PMC4475397
- DOI: 10.15252/embj.201490791
USP18 lack in microglia causes destructive interferonopathy of the mouse brain
Abstract
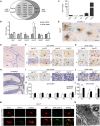
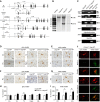
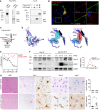
Microglia are tissue macrophages of the central nervous system (CNS) that control tissue homeostasis. Microglia dysregulation is thought to be causal for a group of neuropsychiatric, neurodegenerative and neuroinflammatory diseases, called "microgliopathies". However, how the intracellular stimulation machinery in microglia is controlled is poorly understood. Here, we identified the ubiquitin-specific protease (Usp) 18 in white matter microglia that essentially contributes to microglial quiescence. We further found that microglial Usp18 negatively regulates the activation of Stat1 and concomitant induction of interferon-induced genes, thereby terminating IFN signaling. The Usp18-mediated control was independent from its catalytic activity but instead required the interaction with Ifnar2. Additionally, the absence of Ifnar1 restored microglial activation, indicating a tonic IFN signal which needs to be negatively controlled by Usp18 under non-diseased conditions. These results identify Usp18 as a critical negative regulator of microglia activation and demonstrate a protective role of Usp18 for microglia function by regulating the Ifnar pathway. The findings establish Usp18 as a new molecule preventing destructive microgliopathy.
Keywords: EAE; Usp18; microglia; multiple sclerosis; type I interferon.
© 2015 The Authors.
Figures




Comment in
-
Poised for action: USP18 restrains microglial activation in the white matter.EMBO J. 2015 Jun 12;34(12):1603-5. doi: 10.15252/embj.201591899. Epub 2015 May 13. EMBO J. 2015. PMID: 25971776 Free PMC article.
Similar articles
-
USP18 - a multifunctional component in the interferon response.Biosci Rep. 2018 Nov 15;38(6):BSR20180250. doi: 10.1042/BSR20180250. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30126853 Free PMC article. Review.
-
Microglial Interferon Signaling and White Matter.Neurochem Res. 2017 Sep;42(9):2625-2638. doi: 10.1007/s11064-017-2307-8. Epub 2017 May 25. Neurochem Res. 2017. PMID: 28540600 Free PMC article. Review.
-
Poised for action: USP18 restrains microglial activation in the white matter.EMBO J. 2015 Jun 12;34(12):1603-5. doi: 10.15252/embj.201591899. Epub 2015 May 13. EMBO J. 2015. PMID: 25971776 Free PMC article.
-
Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome.J Exp Med. 2016 Jun 27;213(7):1163-74. doi: 10.1084/jem.20151529. Epub 2016 Jun 20. J Exp Med. 2016. PMID: 27325888 Free PMC article. Clinical Trial.
-
Gene expression profile after knockdown of USP18 in Hepg2.2.15 cells.J Med Virol. 2017 Nov;89(11):1920-1930. doi: 10.1002/jmv.24819. Epub 2017 Aug 28. J Med Virol. 2017. PMID: 28369997
Cited by
-
The Glioblastoma Microenvironment: Morphology, Metabolism, and Molecular Signature of Glial Dynamics to Discover Metabolic Rewiring Sequence.Int J Mol Sci. 2021 Mar 24;22(7):3301. doi: 10.3390/ijms22073301. Int J Mol Sci. 2021. PMID: 33804873 Free PMC article. Review.
-
Do not judge a cell by its cover--diversity of CNS resident, adjoining and infiltrating myeloid cells in inflammation.Semin Immunopathol. 2015 Nov;37(6):591-605. doi: 10.1007/s00281-015-0520-6. Epub 2015 Aug 7. Semin Immunopathol. 2015. PMID: 26251238 Review.
-
USP18 - a multifunctional component in the interferon response.Biosci Rep. 2018 Nov 15;38(6):BSR20180250. doi: 10.1042/BSR20180250. Print 2018 Dec 21. Biosci Rep. 2018. PMID: 30126853 Free PMC article. Review.
-
Microglial senescence contributes to female-biased neuroinflammation in the aging mouse hippocampus: implications for Alzheimer's disease.J Neuroinflammation. 2023 Aug 16;20(1):188. doi: 10.1186/s12974-023-02870-2. J Neuroinflammation. 2023. PMID: 37587511 Free PMC article.
-
Epigenetic Regulation of ZBTB18 Promotes Glioblastoma Progression.Mol Cancer Res. 2017 Aug;15(8):998-1011. doi: 10.1158/1541-7786.MCR-16-0494. Epub 2017 May 16. Mol Cancer Res. 2017. PMID: 28512252 Free PMC article.
References
-
- Basters A, Geurink PP, El OF, Ketscher L, Casutt MS, Krause E, Ovaa H, Knobeloch KP, Fritz G. Molecular characterization of ubiquitin-specific protease 18 reveals substrate specificity for interferon-stimulated gene 15. FEBS J. 2014;281:1918–1928. - PubMed
-
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. - PubMed
-
- Crow YJ. Type I interferonopathies: Mendelian type I interferon up-regulation. Curr Opin Immunol. 2015;32C:7–12. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

