Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection
- PMID: 25896687
- PMCID: PMC4468657
- DOI: 10.1128/AAC.00208-15
Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection
Abstract
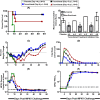
The therapeutic efficacies of smallpox vaccine ACAM2000 and antiviral tecovirimat given alone or in combination starting on day 3 postinfection were compared in a cynomolgus macaque model of lethal monkeypox virus infection. Postexposure administration of ACAM2000 alone did not provide any protection against severe monkeypox disease or mortality. In contrast, postexposure treatment with tecovirimat alone or in combination with ACAM2000 provided full protection. Additionally, tecovirimat treatment delayed until day 4, 5, or 6 postinfection was 83% (days 4 and 5) or 50% (day 6) effective.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge.Vaccine. 2020 Jan 16;38(3):644-654. doi: 10.1016/j.vaccine.2019.10.049. Epub 2019 Oct 31. Vaccine. 2020. PMID: 31677948 Free PMC article.
-
Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection.Nature. 2006 Feb 9;439(7077):745-8. doi: 10.1038/nature04295. Epub 2005 Dec 11. Nature. 2006. PMID: 16341204
-
Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques.J Infect Dis. 2018 Sep 22;218(9):1490-1499. doi: 10.1093/infdis/jiy326. J Infect Dis. 2018. PMID: 29982575 Free PMC article.
-
Treatment and Vaccination for Smallpox and Monkeypox.Adv Exp Med Biol. 2024;1451:301-316. doi: 10.1007/978-3-031-57165-7_19. Adv Exp Med Biol. 2024. PMID: 38801586 Review.
-
New Perspectives on Antimicrobial Agents: Tecovirimat for Treatment of Human Monkeypox Virus.Antimicrob Agents Chemother. 2022 Dec 20;66(12):e0122622. doi: 10.1128/aac.01226-22. Epub 2022 Nov 14. Antimicrob Agents Chemother. 2022. PMID: 36374026 Free PMC article. Review.
Cited by
-
Enhanced Immune Responses in Mice by Combining the Mpox Virus B6R-Protein and Aluminum Hydroxide-CpG Vaccine Adjuvants.Vaccines (Basel). 2024 Jul 15;12(7):776. doi: 10.3390/vaccines12070776. Vaccines (Basel). 2024. PMID: 39066415 Free PMC article.
-
Targeting ectromelia virus and TNF/NF-κB or STAT3 signaling for effective treatment of viral pneumonia.Proc Natl Acad Sci U S A. 2022 Feb 22;119(8):e2112725119. doi: 10.1073/pnas.2112725119. Proc Natl Acad Sci U S A. 2022. PMID: 35177474 Free PMC article.
-
Challenges and Achievements in Prevention and Treatment of Smallpox.Vaccines (Basel). 2018 Jan 29;6(1):8. doi: 10.3390/vaccines6010008. Vaccines (Basel). 2018. PMID: 29382130 Free PMC article. Review.
-
Monkeypox: A comprehensive review of a multifaceted virus.Infect Med (Beijing). 2023 May 12;2(2):74-88. doi: 10.1016/j.imj.2023.04.009. eCollection 2023 Jun. Infect Med (Beijing). 2023. PMID: 38077831 Free PMC article. Review.
-
An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications.Expert Rev Anti Infect Ther. 2021 Mar;19(3):331-344. doi: 10.1080/14787210.2020.1819791. Epub 2020 Sep 15. Expert Rev Anti Infect Ther. 2021. PMID: 32882158 Free PMC article. Review.
References
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/smallpox/9241561106.pdf.
-
- Keckler MS, Reynolds MG, Damon IK, Karem KL. 2013. The effects of post-exposure smallpox vaccination on clinical disease presentation: addressing the data gaps between historical epidemiology and modern surrogate model data. Vaccine 31:5192–5201. doi:10.1016/j.vaccine.2013.08.039. - DOI - PMC - PubMed
-
- U.S. Food and Drug Administration. 2014. Guidance for industry: product development under the Animal Rule. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformati....
-
- Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, DeClercq E, Jones K, Hruby D, Jordan R. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol 79:13139–13149. doi:10.1128/JVI.79.20.13139-13149.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

