Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis
- PMID: 25896688
- PMCID: PMC4468692
- DOI: 10.1128/AAC.00656-15
Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis
Abstract
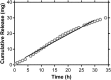
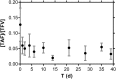
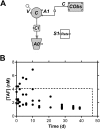
Oral or topical daily administration of antiretroviral (ARV) drugs to HIV-1-negative individuals in vulnerable populations is a promising strategy for HIV-1 prevention. Adherence to the dosing regimen has emerged as a critical factor determining efficacy outcomes of clinical trials. Because adherence to therapy is inversely related to the dosing period, sustained release or long-acting ARV formulations hold significant promise for increasing the effectiveness of HIV-1 preexposure prophylaxis (PrEP) by reducing dosing frequency. A novel, subdermal implant delivering the potent prodrug tenofovir alafenamide (TAF) with controlled, sustained, zero-order (linear) release characteristics is described. A candidate device delivering TAF at 0.92 mg day(-1) in vitro was evaluated in beagle dogs over 40 days for pharmacokinetics and preliminary safety. No adverse events related to treatment with the test article were noted during the course of the study, and no significant, unusual abnormalities were observed. The implant maintained a low systemic exposure to TAF (median, 0.85 ng ml(-1); interquartile range [IQR], 0.60 to 1.50 ng ml(-1)) and tenofovir (TFV; median, 15.0 ng ml(-1); IQR, 8.8 to 23.3 ng ml(-1)), the product of in vivo TAF hydrolysis. High concentrations (median, 512 fmol/10(6) cells over the first 35 days) of the pharmacologically active metabolite, TFV diphosphate, were observed in peripheral blood mononuclear cells at levels over 30 times higher than those associated with HIV-1 PrEP efficacy in humans. Our report on the first sustained-release nucleoside reverse transcriptase inhibitor (NRTI) for systemic delivery demonstrates a successful proof of principle and holds significant promise as a candidate for HIV-1 prophylaxis in vulnerable populations.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

