Bioluminescence for assessing drug potency against nonreplicating Mycobacterium tuberculosis
- PMID: 25896710
- PMCID: PMC4468706
- DOI: 10.1128/AAC.00528-15
Bioluminescence for assessing drug potency against nonreplicating Mycobacterium tuberculosis
Abstract
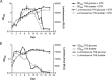
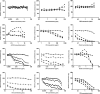
Targeting dormant Mycobacterium tuberculosis represents a challenge to antituberculosis drug discovery programs. We previously reported and validated the use of the streptomycin (STR)-dependent M. tuberculosis 18b strain as a tool for assessing drug potency against nonreplicating bacteria both in vitro and in vivo. In this study, we generated a luminescent 18b strain, named 18b-Lux, by transforming the bacteria with a vector expressing the luxCDABE operon from Photorhabdus luminescens. Luciferase expression was demonstrated under replicating conditions, and, more importantly, luminescence levels significantly above background were detected following STR removal. The sensitivity of STR-starved 18b-Lux to approved and candidate antituberculosis therapeutic agents was evaluated by means of a luciferase assay in a 96-well format. Results mirrored the data obtained with the standard resazurin reduction microplate assay, and the luminescence readout allowed time course assessments of drug efficacy in vitro. Specifically, we proved that bedaquiline, the rifamycins, and sutezolid displayed time-dependent activity against dormant bacteria, while pyrazinamide and SQ109 showed bactericidal effects at the highest concentrations tested. Overall, we established the optimal conditions for an inexpensive, simple, and very sensitive assay with great potential for future applications.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Streptomycin-starved Mycobacterium tuberculosis 18b, a drug discovery tool for latent tuberculosis.Antimicrob Agents Chemother. 2012 Nov;56(11):5782-9. doi: 10.1128/AAC.01125-12. Epub 2012 Aug 27. Antimicrob Agents Chemother. 2012. PMID: 22926567 Free PMC article.
-
Rapid evaluation in whole blood culture of regimens for XDR-TB containing PNU-100480 (sutezolid), TMC207, PA-824, SQ109, and pyrazinamide.PLoS One. 2012;7(1):e30479. doi: 10.1371/journal.pone.0030479. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279595 Free PMC article.
-
Rapid, Semiquantitative Assay To Discriminate among Compounds with Activity against Replicating or Nonreplicating Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2015 Oct;59(10):6521-38. doi: 10.1128/AAC.00803-15. Epub 2015 Aug 3. Antimicrob Agents Chemother. 2015. PMID: 26239979 Free PMC article.
-
In vitro and in vivo activities of three oxazolidinones against nonreplicating Mycobacterium tuberculosis.Antimicrob Agents Chemother. 2014 Jun;58(6):3217-23. doi: 10.1128/AAC.02410-14. Epub 2014 Mar 24. Antimicrob Agents Chemother. 2014. PMID: 24663022 Free PMC article.
-
Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action.Future Microbiol. 2012 Jul;7(7):823-37. doi: 10.2217/fmb.12.56. Future Microbiol. 2012. PMID: 22827305 Free PMC article. Review.
Cited by
-
Tricyclic SpiroLactams Kill Mycobacteria In Vitro and In Vivo by Inhibiting Type II NADH Dehydrogenases.J Med Chem. 2022 Dec 22;65(24):16651-16664. doi: 10.1021/acs.jmedchem.2c01493. Epub 2022 Dec 6. J Med Chem. 2022. PMID: 36473699 Free PMC article.
-
Genomic and transcriptomic analysis of the streptomycin-dependent Mycobacterium tuberculosis strain 18b.BMC Genomics. 2016 Mar 5;17:190. doi: 10.1186/s12864-016-2528-2. BMC Genomics. 2016. PMID: 26944551 Free PMC article.
-
Pharmacokinetics and Target Attainment of SQ109 in Plasma and Human-Like Tuberculosis Lesions in Rabbits.Antimicrob Agents Chemother. 2021 Aug 17;65(9):e0002421. doi: 10.1128/AAC.00024-21. Epub 2021 Aug 17. Antimicrob Agents Chemother. 2021. PMID: 34228540 Free PMC article.
-
Phosphoglucomutase A-mediated metabolic adaptation is essential for antibiotic and disease persistence in Mycobacterium tuberculosis.mSystems. 2025 Jul 22;10(7):e0042025. doi: 10.1128/msystems.00420-25. Epub 2025 Jun 30. mSystems. 2025. PMID: 40586596 Free PMC article.
-
Re-Understanding the Mechanisms of Action of the Anti-Mycobacterial Drug Bedaquiline.Antibiotics (Basel). 2019 Dec 11;8(4):261. doi: 10.3390/antibiotics8040261. Antibiotics (Basel). 2019. PMID: 31835707 Free PMC article. Review.
References
-
- World Health Organization. 2014. Tuberculosis fact sheet. World Health Organization, Geneva, Switzerland.
-
- Zhang M, Sala C, Dhar N, Vocat A, Sambandamurthy VK, Sharma S, Marriner G, Balasubramanian V, Cole ST. 2014. In vitro and in vivo activities of three oxazolidinones against nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 58:3217–3223. doi:10.1128/AAC.02410-14. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

