The Cyclic AMP-Vfr Signaling Pathway in Pseudomonas aeruginosa Is Inhibited by Cyclic Di-GMP
- PMID: 25897033
- PMCID: PMC4455276
- DOI: 10.1128/JB.00193-15
The Cyclic AMP-Vfr Signaling Pathway in Pseudomonas aeruginosa Is Inhibited by Cyclic Di-GMP
Erratum in
-
Erratum for Almblad et al., The Cyclic AMP-Vfr Signaling Pathway in Pseudomonas aeruginosa Is Inhibited by Cyclic Di-GMP.J Bacteriol. 2015 Aug;197(16):2731. doi: 10.1128/JB.00493-15. J Bacteriol. 2015. PMID: 26187287 Free PMC article. No abstract available.
Abstract
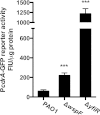
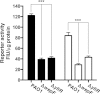
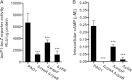
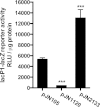
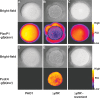
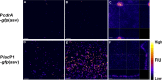
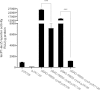
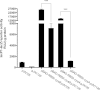
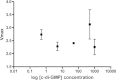
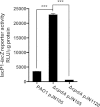
The opportunistic human pathogen Pseudomonas aeruginosa expresses numerous acute virulence factors in the initial phase of infection, and during long-term colonization it undergoes adaptations that optimize survival in the human host. Adaptive changes that often occur during chronic infection give rise to rugose small colony variants (RSCVs), which are hyper-biofilm-forming mutants that commonly possess mutations that increase production of the biofilm-promoting secondary messenger cyclic di-GMP (c-di-GMP). We show that RSCVs display a decreased production of acute virulence factors as a direct result of elevated c-di-GMP content. Overproduction of c-di-GMP causes a decrease in the transcription of virulence factor genes that are regulated by the global virulence regulator Vfr. The low level of Vfr-dependent transcription is caused by a low level of its coactivator, cyclic AMP (cAMP), which is decreased in response to a high level of c-di-GMP. Mutations that cause reversion of the RSCV phenotype concomitantly reactivate Vfr-cAMP signaling. Attempts to uncover the mechanism underlying the observed c-di-GMP-mediated lowering of cAMP content provided evidence that it is not caused by inhibition of adenylate cyclase production or activity and that it is not caused by activation of cAMP phosphodiesterase activity. In addition to the studies of the RSCVs, we present evidence that the deeper layers of wild-type P. aeruginosa biofilms have high c-di-GMP levels and low cAMP levels.
Importance: Our work suggests that cross talk between c-di-GMP and cAMP signaling pathways results in downregulation of acute virulence factors in P. aeruginosa biofilm infections. Knowledge about this cross-regulation adds to our understanding of virulence traits and immune evasion by P. aeruginosa in chronic infections and may provide new approaches to eradicate biofilm infections.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Smith EE, Buckley DG, Wu Z, Saenphimmachak C, Hoffman LR, D'Argenio DA, Miller SI, Ramsey BW, Speert DP, Moskowitz SM, Burns JL, Kaul R, Olson MV. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A 103:8487–8492. doi: 10.1073/pnas.0602138103. - DOI - PMC - PubMed
-
- Mishra M, Byrd MS, Sergeant S, Azad AK, Parsek MR, McPhail L, Schlesinger LS, Wozniak DJ. 2012. Pseudomonas aeruginosa Psl polysaccharide reduces neutrophil phagocytosis and the oxidative response by limiting complement-mediated opsonization. Cell Microbiol 14:95–106. doi: 10.1111/j.1462-5822.2011.01704.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

