TREM-2 promotes macrophage survival and lung disease after respiratory viral infection
- PMID: 25897174
- PMCID: PMC4419356
- DOI: 10.1084/jem.20141732
TREM-2 promotes macrophage survival and lung disease after respiratory viral infection
Abstract
Viral infections and type 2 immune responses are thought to be critical for the development of chronic respiratory disease, but the link between these events needs to be better defined. Here, we study a mouse model in which infection with a mouse parainfluenza virus known as Sendai virus (SeV) leads to long-term activation of innate immune cells that drive IL-13-dependent lung disease. We find that chronic postviral disease (signified by formation of excess airway mucus and accumulation of M2-differentiating lung macrophages) requires macrophage expression of triggering receptor expressed on myeloid cells-2 (TREM-2). Analysis of mechanism shows that viral replication increases lung macrophage levels of intracellular and cell surface TREM-2, and this action prevents macrophage apoptosis that would otherwise occur during the acute illness (5-12 d after inoculation). However, the largest increases in TREM-2 levels are found as the soluble form (sTREM-2) long after clearance of infection (49 d after inoculation). At this time, IL-13 and the adapter protein DAP12 promote TREM-2 cleavage to sTREM-2 that is unexpectedly active in preventing macrophage apoptosis. The results thereby define an unprecedented mechanism for a feed-forward expansion of lung macrophages (with IL-13 production and consequent M2 differentiation) that further explains how acute infection leads to chronic inflammatory disease.
© 2015 Wu et al.
Figures


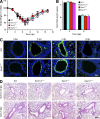
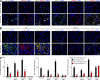
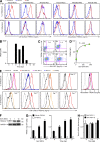

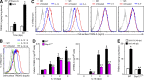



References
-
- Bleharski J.R., Kiessler V., Buonsanti C., Sieling P.A., Stenger S., Colonna M., and Modlin R.L.. 2003. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J. Immunol. 170:3812–3818 10.4049/jimmunol.170.7.3812 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL029594/HL/NHLBI NIH HHS/United States
- P01-HL-029594/HL/NHLBI NIH HHS/United States
- R01-HL-073159/HL/NHLBI NIH HHS/United States
- P50-HL-084922/HL/NHLBI NIH HHS/United States
- R01 HL073159/HL/NHLBI NIH HHS/United States
- R01 HL121791/HL/NHLBI NIH HHS/United States
- U19 AI070489/AI/NIAID NIH HHS/United States
- R01 HL119813/HL/NHLBI NIH HHS/United States
- T32 HL007317/HL/NHLBI NIH HHS/United States
- R01-HL-121791/HL/NHLBI NIH HHS/United States
- T32-HL-007317/HL/NHLBI NIH HHS/United States
- U19-AI07048/AI/NIAID NIH HHS/United States
- P50 HL084922/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

