Aclidinium bromide/formoterol fixed-dose combination therapy for COPD: the evidence to date
- PMID: 25897208
- PMCID: PMC4396584
- DOI: 10.2147/DDDT.S53150
Aclidinium bromide/formoterol fixed-dose combination therapy for COPD: the evidence to date
Abstract
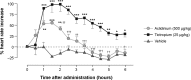
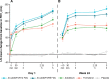
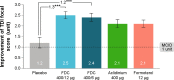
The quest for the right combination of bronchodilators with different mechanisms of action such as long-acting muscarinic antagonists and long-acting β-agonists in the management of stable moderate-to-severe chronic obstructive pulmonary disease (COPD) is a topic of intense research activity currently, given the rising morbidity and mortality due to this disease. The fixed-dose combination of aclidinium bromide and formoterol fumarate in a single inhaler seems to offer superior advantages over either drugs given alone or as separate inhalers concurrently. Since the fixed-dose combination needs to be given twice daily, it is likely to achieve control of symptoms most crucial to the quality of life in COPD, namely, the morning hours. This is reflected in significant trough FEV1 (forced expiratory volume in 1 second) improvements after the dose. This paper reviews the various studies related to this combination put in the perspective of its safety and efficacy and potential benefits over other therapeutic options. However, there is a dearth of data on the long-term safety and efficacy in terms of improvement in lung function. This combination could emerge as an excellent option in the management of stable COPD if data on exacerbation rates and patient-reported outcomes become available from longer-term studies. Moreover, we need some more studies to define the ideal phenotype of COPD best suited for the use of this combination.
Keywords: COPD; aclidinium; bronchodilators; combination therapy; formoterol; lung function.
Figures


Similar articles
-
Long-term safety of aclidinium bromide/formoterol fumarate fixed-dose combination: Results of a randomized 1-year trial in patients with COPD.Respir Med. 2016 Jul;116:41-8. doi: 10.1016/j.rmed.2016.05.007. Epub 2016 May 7. Respir Med. 2016. PMID: 27296819 Clinical Trial.
-
Clinicopharmacological profile of the fixed-dose combination of aclidinium bromide and formoterol fumarate in the management of chronic obstructive pulmonary disease.Ther Adv Respir Dis. 2015 Apr;9(2):56-68. doi: 10.1177/1753465815575254. Epub 2015 Mar 9. Ther Adv Respir Dis. 2015. PMID: 25754881 Review.
-
Symptom variability and control in COPD: Advantages of dual bronchodilation therapy.Respir Med. 2017 Apr;125:49-56. doi: 10.1016/j.rmed.2017.03.001. Epub 2017 Mar 2. Respir Med. 2017. PMID: 28340862 Review.
-
Aclidinium bromide and formoterol fumarate for the maintenance treatment of chronic obstructive pulmonary disease.Expert Rev Clin Pharmacol. 2020 Feb;13(2):103-113. doi: 10.1080/17512433.2020.1717334. Epub 2020 Jan 29. Expert Rev Clin Pharmacol. 2020. PMID: 31951778 Review.
-
Dual bronchodilator therapy with aclidinium bromide/formoterol fumarate for chronic obstructive pulmonary disease.Expert Rev Respir Med. 2015 Oct;9(5):519-32. doi: 10.1586/17476348.2015.1081065. Epub 2015 Aug 26. Expert Rev Respir Med. 2015. PMID: 26366803 Review.
Cited by
-
Effects of Low-Dose and Long-Term Treatment with Erythromycin on Interleukin-17 and Interleukin-23 in Peripheral Blood and Induced Sputum in Patients with Stable Chronic Obstructive Pulmonary Disease.Mediators Inflamm. 2016;2016:4173962. doi: 10.1155/2016/4173962. Epub 2016 Apr 3. Mediators Inflamm. 2016. PMID: 27127346 Free PMC article. Clinical Trial.
-
Specific role of combination aclidinium: formoterol in the treatment of chronic obstructive pulmonary disease.Int J Chron Obstruct Pulmon Dis. 2016 Jan 5;11:73-9. doi: 10.2147/COPD.S78000. eCollection 2016. Int J Chron Obstruct Pulmon Dis. 2016. PMID: 26792987 Free PMC article. Review.
-
Inhaled treatment for chronic obstructive pulmonary disease: what's new and how does it fit?QJM. 2016 Aug;109(8):505-12. doi: 10.1093/qjmed/hcv212. Epub 2015 Nov 11. QJM. 2016. PMID: 26559079 Free PMC article. Review.
-
New combinations in the treatment of COPD: rationale for aclidinium-formoterol.Ther Clin Risk Manag. 2016 Feb 15;12:209-15. doi: 10.2147/TCRM.S82034. eCollection 2016. Ther Clin Risk Manag. 2016. PMID: 26929634 Free PMC article. Review.
References
-
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. - PubMed
-
- Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. - PubMed
-
- Chapman KR, Mannino DM, Soriano JB, et al. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27(1):188–207. - PubMed
-
- Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

