HIV protease inhibitors: a review of molecular selectivity and toxicity
- PMID: 25897264
- PMCID: PMC4396582
- DOI: 10.2147/HIV.S79956
HIV protease inhibitors: a review of molecular selectivity and toxicity
Abstract
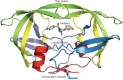
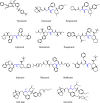
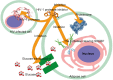
Highly active antiretroviral therapy (HAART) is recognized as the most effective treatment method for AIDS, and protease inhibitors play a very important role in HAART. However, poor bioavailability and unbearable toxicity are their common disadvantages. Thus, the development of safer and potentially promising protease inhibitors is eagerly needed. In this review, we introduced the chemical characteristics and associated side effects of HIV protease inhibitors, as well as the possible off-target mechanisms causing the side effects. From the chemical structures of HIV protease inhibitors and their possible off-target molecules, we could obtain hints for optimizing the molecular selectivity of the inhibitors, to provide help in the design of new compounds with enhanced bioavailability and reduced side effects.
Keywords: glucose transporter-4; off-target; proteasome; side effect.
Figures
References
-
- UNAIDS . Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. Geneva: UNAIDS; 2010.
-
- Bozzette SA, Ake CF, Tam HK, Chang SW, Louis TA. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348(8):702–710. - PubMed
-
- Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49(Suppl 2):S79–S85. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases