The Effects of Antenatal Corticosteroids on Short- and Long-Term Outcomes in Small-for-Gestational-Age Infants
- PMID: 25897289
- PMCID: PMC4402431
- DOI: 10.7150/ijms.11523
The Effects of Antenatal Corticosteroids on Short- and Long-Term Outcomes in Small-for-Gestational-Age Infants
Abstract
Aim: To evaluate the effect of antenatal corticosteroids (ANS) on short- and long-term outcomes in small-for-gestational age (SGA) infants.
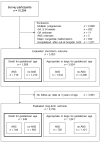
Methods: A retrospective database analysis was performed. A total of 1,931 single infants (birth weight <1,500 g) born at a gestational age between 22 weeks and 33 weeks 6 days who were determined to be SGA registered in the Neonatal Research Network Database in Japan between 2003 and 2007 were evaluated for short-term outcome and long-term outcome.
Results: ANS was administered to a total of 719 infants (37%) in the short-term outcome evaluation group and 344 infants (36%) in the long-term outcome evaluation group. There were no significant differences between the ANS group and the no-ANS group for primary short-term outcome (adjusted odds ratio (OR) 0.73; 95% confidence interval (CI) 0.45-1.20; P-value 0.22) or primary long-term outcome (adjusted OR 0.69; 95% CI 0.40-1.17; P-value 0.17).
Conclusions: Our results show that ANS does not affect short- or long-term outcome in SGA infants when the birth weight is less than 1500 g. This study strongly suggests that administration of ANS resulted in few benefits for preterm FGR fetuses.
Keywords: fetal growth restriction; glucocorticoids; infant; infant mortality; premature birth; small for gestational age.
Conflict of interest statement
Competing Interests: This study was partly supported by a grant from the Ministry of Health, Labor and Welfare, Japan.
Figures
References
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatr. 1972;50:515–525. - PubMed
-
- Crowley PA. Antenatal corticosteroid therapy: a meta-analysis of the randomized trials, 1972 to 1994. Am J Obstet Gynecol. 1995;173:322–335. [DOI:10.1016/0002-9378(95)90222-8] - PubMed
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;19:CD004454. CD004454 [DOI:10.1002/14651858.CD004454.pub2] - PubMed
-
- Elimian A, Verma U, Canterino J. et al. Effectiveness of antenatal steroids in obstetric subgroups. Obstet Gynecol. 1999;93:174–179. [DOI:10.1016/S0029-7844(98)00400-1] - PubMed
-
- Morrison JL, Orgeig S. Antenatal glucocorticoid treatment of the growth-restricted fetus: benefit or cost? Reprod Sci. 2009;16:527–538. [DOI:10.1177/1933719109332821] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous