Blue blood on ice: modulated blood oxygen transport facilitates cold compensation and eurythermy in an Antarctic octopod
- PMID: 25897316
- PMCID: PMC4403823
- DOI: 10.1186/s12983-015-0097-x
Blue blood on ice: modulated blood oxygen transport facilitates cold compensation and eurythermy in an Antarctic octopod
Abstract
Introduction: The Antarctic Ocean hosts a rich and diverse fauna despite inhospitable temperatures close to freezing, which require specialist adaptations to sustain animal activity and various underlying body functions. While oxygen transport has been suggested to be key in setting thermal tolerance in warmer climates, this constraint is relaxed in Antarctic fishes and crustaceans, due to high levels of dissolved oxygen. Less is known about how other Antarctic ectotherms cope with temperatures near zero, particularly the more active invertebrates like the abundant octopods. A continued reliance on the highly specialised blood oxygen transport system of cephalopods may concur with functional constraints at cold temperatures. We therefore analysed the octopod's central oxygen transport component, the blue blood pigment haemocyanin, to unravel strategies that sustain oxygen supply at cold temperatures.
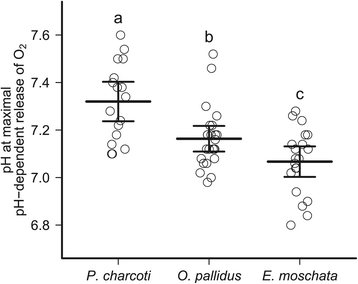
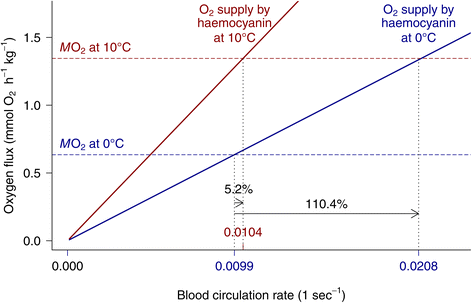
Results: To identify adaptive compensation of blood oxygen transport in octopods from different climatic regions, we compared haemocyanin oxygen binding properties, oxygen carrying capacities as well as haemolymph protein and ion composition between the Antarctic octopod Pareledone charcoti, the South-east Australian Octopus pallidus and the Mediterranean Eledone moschata. In the Antarctic Pareledone charcoti at 0°C, oxygen unloading by haemocyanin was poor but supported by high levels of dissolved oxygen. However, lower oxygen affinity and higher oxygen carrying capacity compared to warm water octopods, still enabled significant contribution of haemocyanin to oxygen transport at 0°C. At warmer temperatures, haemocyanin of Pareledone charcoti releases most of the bound oxygen, supporting oxygen supply at 10°C. In warm water octopods, increasing oxygen affinities reduce the ability to release oxygen from haemocyanin at colder temperatures. Though, unlike Eledone moschata, Octopus pallidus attenuated this increase below 15°C.
Conclusions: Adjustments of haemocyanin physiological function and haemocyanin concentrations but also high dissolved oxygen concentrations support oxygen supply in the Antarctic octopus Pareledone charcoti at near freezing temperatures. Increased oxygen supply by haemocyanin at warmer temperatures supports extended warm tolerance and thus eurythermy of Pareledone charcoti. Limited haemocyanin function towards colder temperatures in Antarctic and warm water octopods highlights the general role of haemocyanin oxygen transport in constraining cold tolerance in octopods.
Keywords: Cephalopod; Diffusion chamber; Eledone moschata; Haemocyanin; Hemocyanin; Octopus pallidus; Oxygen affinity; Oxygen carrying capacity; Pareledone charcoti.
Figures





Similar articles
-
Energy balance and cold adaptation in the octopus Pareledone charcoti.J Exp Mar Biol Ecol. 2000 Mar 15;245(2):197-214. doi: 10.1016/s0022-0981(99)00161-6. J Exp Mar Biol Ecol. 2000. PMID: 10699210
-
Positive selection in octopus haemocyanin indicates functional links to temperature adaptation.BMC Evol Biol. 2015 Jul 5;15:133. doi: 10.1186/s12862-015-0411-4. BMC Evol Biol. 2015. PMID: 26142723 Free PMC article.
-
Temperature effects on hemocyanin oxygen binding in an antarctic cephalopod.Biol Bull. 2001 Feb;200(1):67-76. doi: 10.2307/1543086. Biol Bull. 2001. PMID: 11249213
-
Antarctic notothenioid fish: what are the future consequences of 'losses' and 'gains' acquired during long-term evolution at cold and stable temperatures?J Exp Biol. 2015 Jun;218(Pt 12):1834-45. doi: 10.1242/jeb.116129. J Exp Biol. 2015. PMID: 26085661 Review.
-
Biochemical adaptations of notothenioid fishes: comparisons between cold temperate South American and New Zealand species and Antarctic species.Comp Biochem Physiol A Mol Integr Physiol. 2007 Jul;147(3):799-807. doi: 10.1016/j.cbpa.2006.09.028. Epub 2006 Dec 5. Comp Biochem Physiol A Mol Integr Physiol. 2007. PMID: 17293146 Review.
Cited by
-
Expanding our scientific horizons: utilization of unique model organisms in biological research.EMBO J. 2017 Aug 15;36(16):2311-2314. doi: 10.15252/embj.201797640. Epub 2017 Jul 10. EMBO J. 2017. PMID: 28694243 Free PMC article.
-
The first global deep-sea stable isotope assessment reveals the unique trophic ecology of Vampire Squid Vampyroteuthis infernalis (Cephalopoda).Sci Rep. 2019 Dec 13;9(1):19099. doi: 10.1038/s41598-019-55719-1. Sci Rep. 2019. PMID: 31836823 Free PMC article.
-
Life histories of Antarctic incirrate octopods (Cephalopoda: Octopoda).PLoS One. 2019 Jul 11;14(7):e0219694. doi: 10.1371/journal.pone.0219694. eCollection 2019. PLoS One. 2019. PMID: 31295339 Free PMC article.
-
Thermal sensitivity links to cellular cardiac decline in three spiny lobsters.Sci Rep. 2020 Jan 14;10(1):202. doi: 10.1038/s41598-019-56794-0. Sci Rep. 2020. PMID: 31937868 Free PMC article.
-
The Evolution of Hemocyanin Genes in Caenogastropoda: Gene Duplications and Intron Accumulation in Highly Diverse Gastropods.J Mol Evol. 2021 Dec;89(9-10):639-655. doi: 10.1007/s00239-021-10036-y. Epub 2021 Nov 10. J Mol Evol. 2021. PMID: 34757470 Free PMC article.
References
-
- Klinck JM, Hofmann EE, Beardsley RC, Salihoglu B, Howard S. Water-mass properties and circulation on the west Antarctic Peninsula Continental Shelf in Austral Fall and Winter 2001. Deep-Sea Res Pt II. 2004;51:1925–46.
-
- Jacobs SS, Amos AF, Bruchhausen PM. Ross sea oceanography and antarctic bottom water formation. Deep-Sea Res Oceanogr Abstr. 1970;17:935–62.
-
- Sidell BD, O'Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol. 2006;209:1791–802. - PubMed
-
- Clarke A, Johnston NM. Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol. 1999;68:893–905.
LinkOut - more resources
Full Text Sources
Other Literature Sources

