Interplay between Rab27a effectors in pancreatic β-cells
- PMID: 25897360
- PMCID: PMC4398906
- DOI: 10.4239/wjd.v6.i3.508
Interplay between Rab27a effectors in pancreatic β-cells
Abstract
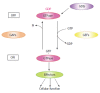
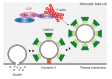
The small GTPase Rab27a is a member of the Rab family that is involved in membrane trafficking in various kinds of cells. Rab27a has GTP- and GDP-bound forms, and their interconversion regulates intracellular signaling pathways. Typically, only a GTP-bound GTPase binds its specific effectors with the resulting downstream signals controlling specific cellular functions. We previously identified novel Rab27a-interacting proteins. Surprisingly, some of these proteins interacted with GDP-bound Rab27a. The present study reviews recent progress in our understanding of the roles of Rab27a and its effectors in the secretory process. In pancreatic β-cells, GTP-bound Rab27a regulates insulin secretion at the pre-exocytotic stages via its GTP-specific effectors such as Exophilin8/Slac2-c/MyRIP and Slp4/Granuphilin. Glucose stimulation causes insulin exocytosis. Glucose stimulation also converts Rab27a from its GTP- to its GDP-bound form. GDP-bound Rab27a interacts with GDP-specific effectors and controls endocytosis of the secretory membrane. Thus, Rab27a cycling between GTP- and GDP-bound forms synchronizes with the recycling of secretory membrane to re-use the membrane and keep the β-cell volume constant.
Keywords: Coronin 3; Endocytosis; Exocytosis; Glucose; IQGAP1; Insulin; Rab27a; Small GTPase.
Figures



Similar articles
-
GTP- and GDP-Dependent Rab27a Effectors in Pancreatic Beta-Cells.Biol Pharm Bull. 2015;38(5):663-8. doi: 10.1248/bpb.b14-00886. Biol Pharm Bull. 2015. PMID: 25947911 Review.
-
Rab27a, actin and beta-cell endocytosis.Endocr J. 2011;58(1):1-6. doi: 10.1507/endocrj.k10e-391. Epub 2010 Dec 23. Endocr J. 2011. PMID: 21187662 Review.
-
The GDP-dependent Rab27a effector coronin 3 controls endocytosis of secretory membrane in insulin-secreting cell lines.J Cell Sci. 2008 Sep 15;121(Pt 18):3092-8. doi: 10.1242/jcs.030544. J Cell Sci. 2008. PMID: 18768935
-
Rab27a in pancreatic beta-cells, a busy protein in membrane trafficking.Prog Biophys Mol Biol. 2011 Nov;107(2):219-23. doi: 10.1016/j.pbiomolbio.2011.06.016. Epub 2011 Jul 7. Prog Biophys Mol Biol. 2011. PMID: 21762718 Review.
-
GDP-bound Rab27a regulates clathrin disassembly through HSPA8 after insulin secretion.Arch Biochem Biophys. 2023 Nov;749:109789. doi: 10.1016/j.abb.2023.109789. Epub 2023 Oct 16. Arch Biochem Biophys. 2023. PMID: 37852426
Cited by
-
A conserved electrostatic membrane-binding surface in synaptotagmin-like proteins revealed using molecular phylogenetic analysis and homology modeling.Protein Sci. 2024 Jan;33(1):e4850. doi: 10.1002/pro.4850. Protein Sci. 2024. PMID: 38038838 Free PMC article.
-
A Conserved Electrostatic Membrane-Binding Surface in Synaptotagmin-Like Proteins Revealed Using Molecular Phylogenetic Analysis and Homology Modeling.bioRxiv [Preprint]. 2023 Oct 29:2023.07.13.548768. doi: 10.1101/2023.07.13.548768. bioRxiv. 2023. Update in: Protein Sci. 2024 Jan;33(1):e4850. doi: 10.1002/pro.4850. PMID: 37502952 Free PMC article. Updated. Preprint.
-
Emerging Roles of Small GTPases in Islet β-Cell Function.Cells. 2021 Jun 15;10(6):1503. doi: 10.3390/cells10061503. Cells. 2021. PMID: 34203728 Free PMC article. Review.
-
Effects of silencing Rab27a gene on biological characteristics and chemosensitivity of non-small cell lung cancer.Oncotarget. 2017 Oct 10;8(55):94481-94492. doi: 10.18632/oncotarget.21782. eCollection 2017 Nov 7. Oncotarget. 2017. PMID: 29212243 Free PMC article.
-
Lysosomal integral membrane protein Sidt2 plays a vital role in insulin secretion.Int J Clin Exp Pathol. 2015 Dec 1;8(12):15622-31. eCollection 2015. Int J Clin Exp Pathol. 2015. PMID: 26884831 Free PMC article.
References
-
- Ashcroft FM, Rorsman P. Molecular defects in insulin secretion in type-2 diabetes. Rev Endocr Metab Disord. 2004;5:135–142. - PubMed
-
- Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–4058. - PubMed
-
- Wennerberg K, Rossman KL, Der CJ. The Ras superfamily at a glance. J Cell Sci. 2005;118:843–846. - PubMed
-
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. - PubMed
-
- Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

