Synergistic enhancement of 5-fluorouracil cytotoxicity by deoxyuridine analogs in cancer cells
- PMID: 25897430
- PMCID: PMC4394133
- DOI: 10.18632/oncoscience.125
Synergistic enhancement of 5-fluorouracil cytotoxicity by deoxyuridine analogs in cancer cells
Abstract
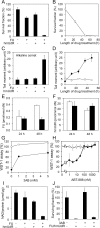
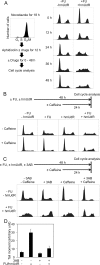
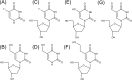
5-Fluorouracil (FU) is a halogenated nucleobase analog that is widely used in chemotherapy. Here we show that 5-hydroxymethyl-2'-deoxyuridine (hmUdR) synergistically enhances the activity of FU in cell lines derived from solid tumors but not normal tissues. While the cytotoxicity of FU and hmUdR was not directly related to the amount of the modified bases incorporated into cellular DNA, incubation with this combination resulted in dramatic increase in the number of single strand breaks in replicating cancer cells, leading to NAD-depletion as consequence of poly(ADP-ribose) synthesis and S phase arrest. Cell death resulting from the base/nucleoside combination did not occur by apoptosis, autophagy or necroptosis. Instead, the cells die via necrosis as a result of NAD depletion. The FU-related nucleoside analog, 5-fluoro-2'-deoxyuridine, also displayed synergy with hmUdR, whereas hmUdR could not be replaced by 5-hydroxymethyluracil. Among other 5-modified deoxyuridine analogs tested, 5-formyl-2'-deoxyuridine and, to a lesser extent, 5-hydroxy-2'-deoxyuridine, also acted synergistically with FU, whereas 5-hydroxyethyl-2'-deoxyuridine did not. Together, our results have revealed an unexpected synergistic interaction between deoxyuridine analogs and FU in a cancer cell-specific manner, and suggest that these novel base/nucleoside combinations could be developed into improved FU-based chemotherapies.
Keywords: 5-Fluorouracil; Cytotoxicity; Deoxyuridine Analog; Synergy.
Conflict of interest statement
Two patent applications related to this work were submitted: One by Yoshihiro Matsumoto as an inventor; another by Yoshihiro Matsumoto, Alan E. Tomkinson and Hiroshi Ide as co-inventors. There is no other conflict of interest related to this work.
Figures



Similar articles
-
Cytotoxic mechanisms of 5-fluoropyrimidines. Relationships with poly(ADP-ribose) polymerase activity, DNA strand breakage and incorporation into nucleic acids.Biochem Pharmacol. 1993 Jul 20;46(2):205-11. doi: 10.1016/0006-2952(93)90405-l. Biochem Pharmacol. 1993. PMID: 8347142
-
Potentiation of the sensitivity of renal cell carcinoma cells to TRAIL-mediated apoptosis by subtoxic concentrations of 5-fluorouracil.Eur J Cancer. 2002 Jan;38(1):167-76. doi: 10.1016/s0959-8049(01)00339-2. Eur J Cancer. 2002. PMID: 11750847
-
Oridonin enhances the cytotoxicity of 5-FU in renal carcinoma cells by inducting necroptotic death.Biomed Pharmacother. 2018 Oct;106:175-182. doi: 10.1016/j.biopha.2018.06.111. Epub 2018 Jun 27. Biomed Pharmacother. 2018. PMID: 29958141
-
Biochemical modulation of fluorouracil by dipyridamole: preclinical and clinical experience.Semin Oncol. 1992 Apr;19(2 Suppl 3):56-65. Semin Oncol. 1992. PMID: 1557658 Review.
-
DNA repair, ADP-ribosylation and pyridine nucleotide metabolism as targets for cancer chemotherapy.Anticancer Drug Des. 1987 Oct;2(2):203-9. Anticancer Drug Des. 1987. PMID: 3329525 Review.
Cited by
-
Incorporation and Repair of Epigenetic Intermediates as Potential Chemotherapy Agents.Molecules. 2025 Aug 1;30(15):3239. doi: 10.3390/molecules30153239. Molecules. 2025. PMID: 40807411 Free PMC article.
References
-
- Grem JL. 5-Fluorouracil: forty-plus and still ticking. A review of its preclinical and clinical development. Investigational New Drugs. 2000;18:299–313. - PubMed
-
- O'Connor OA. Pharmacological Modulation of Fluoropyrimidines: Building on the Lessons of the Past. In: Schwartz GK, editor. Combination Cancer Therapy: Modulators and Potentiators. Totowa, NJ: Humana Press; 2005. pp. 133–174.
-
- Yoshida K, Yamaguchi K, Osada S, Kawaguchi Y, Takahashi T, Sakashita F, Tanaka Y. Challenge for a better combination with basic evidence. Int J Clin Oncol. 2008;13:212–219. - PubMed
-
- Lewis HL, Muhleman DR, Ward JF. Serologic assay of DNA base damage. I. 5-Hydroxymethyldeoxyuridine, a radiation product of thymidine. Radiation Research. 1978;75:305–316. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

