Bone morphogenetic protein 4 (BMP-4) and epidermal growth factor (EGF) inhibit metalloproteinase-9 (MMP-9) expression in cancer cells
- PMID: 25897433
- PMCID: PMC4394136
- DOI: 10.18632/oncoscience.144
Bone morphogenetic protein 4 (BMP-4) and epidermal growth factor (EGF) inhibit metalloproteinase-9 (MMP-9) expression in cancer cells
Abstract
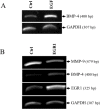
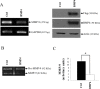
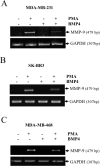
Matrix metalloproteinase-9 (MMP-9) plays a central role in the progression of the cancer. While a large number of studies have contributed to our understanding of the molecular mechanisms responsible for upregulating MMP-9 gene expression in normal and cancer cells, our knowledge on the signals that suppress MMP-9 expression is much more limited. Here, we report that EGF and BMP-4 cooperate to inhibit MMP-9 expression in cancer cells. Treatment with EGF reduces the expression of MMP-9 at both mRNA while augmenting BMP-4 expression. Interestingly, recombinant BMP-4 suppressed constitutive and PMA-induced MMP-9 expression in both fibrosarcoma and breast cancer cells. Addition of gremlin a natural inhibitor of BMP-4, inhibited the suppression of MMP-9 by EGF. The suppression of MMP-9 by BMP-4 likely occurs at the transcriptional level since BMP-4 suppressed MMP-9 mRNA expression and activation of a reporter vector encoding the human MMP-9 promoter. The suppressive effect of BMP-4 occurs via Smad1/5/8 and is specific since BMP-4 did not inhibit MMP-2 while BMP-2 was ineffective in suppressing MMP-9. Taken together, these results are consistent with a new paradigm for the role of EGF and BMPs in controlling MMP gene expression in cancer cells.
Conflict of interest statement
The authors of this manuscript have no conflicts of interest to declare.
Figures




References
-
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. - PubMed
-
- Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. - PubMed
-
- Chicoine E, Esteve PO, Robledo O, Van Themsche C, Potworowski EF, St-Pierre Y. Evidence for the role of promoter methylation in the regulation of MMP-9 gene expression. Biochem Biophys Res Commun. 2002;297:765–772. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

