Defining Potential Vaccine Targets of Haemophilus ducreyi Trimeric Autotransporter Adhesin DsrA
- PMID: 25897604
- PMCID: PMC4410285
- DOI: 10.1089/mab.2014.0054
Defining Potential Vaccine Targets of Haemophilus ducreyi Trimeric Autotransporter Adhesin DsrA
Abstract
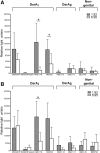
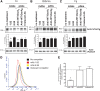
Haemophilus ducreyi is the causative agent of the sexually transmitted genital ulcer disease chancroid. Strains of H. ducreyi are grouped in two classes (I and II) based on genotypic and phenotypic differences, including those found in DsrA, an outer membrane protein belonging to the family of multifunctional trimeric autotransporter adhesins. DsrA is a key serum resistance factor of H. ducreyi that prevents binding of natural IgM at the bacterial surface and functions as an adhesin to fibronectin, fibrinogen, vitronectin, and human keratinocytes. Monoclonal antibodies (MAbs) were developed to recombinant DsrA (DsrA(I)) from prototypical class I strain 35000HP to define targets for vaccine and/or therapeutics. Two anti-DsrAI MAbs bound monomers and multimers of DsrA from genital and non-genital/cutaneous H. ducreyi strains in a Western blot and reacted to the surface of the genital strains; however, these MAbs did not recognize denatured or native DsrA from class II strains. In a modified extracellular matrix protein binding assay using viable H. ducreyi, one of the MAbs partially inhibited binding of fibronectin, fibrinogen, and vitronectin to class I H. ducreyi strain 35000HP, suggesting a role for anti-DsrA antibodies in preventing binding of H. ducreyi to extracellular matrix proteins. Standard ELISA and surface plasmon resonance using a peptide library representing full-length, mature DsrAI revealed the smallest nominal epitope bound by one of the MAbs to be MEQNTHNINKLS. Taken together, our findings suggest that this epitope is a potential target for an H. ducreyi vaccine.
Figures



References
-
- Bong CT, Bauer ME, and Spinola SM: Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microbes Infect 2002;4:1141–1148 - PubMed
-
- Plummer FA, Simonsen JN, Cameron DW, Ndinya-Achola JO, Kreiss JK, Gakinya MN, Waiyaki P, Cheang M, Piot P, Ronald AR, and Ngugi EN: Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis 1991;163:233–239 - PubMed
-
- Wasserheit J: Epidemiological survey. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis 1992;19:61–77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

